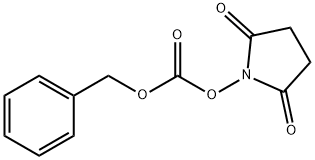
N-(Benzyloxycarbonyloxy)succinimide (Cbz-OSu) is a common reagent for the carboxybenzyl protection of amines. This reaction is one of the key synthetic steps in the synthesis of:
- Enantiomers of cyclic methionine analogs viz, (R)-and (S)-3-aminotetrahydrothiophene- 3-carboxylic acid.
- 1′-H-Spiro-(indoline-3,4′-piperidine) and its derivatives.
- Total synthesis of (-)-diazonamide A. and (-)-sanglifehrin A.
Cbz-OSu is widely employed to protect amino acid residues in peptide synthesis. It can also be used in
N-trans diprotection of cyclen regioselectively.