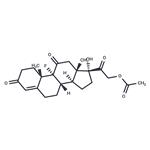
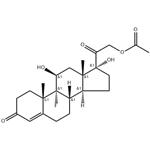
Fludrocortisone acetate is the acetate form of the synthetic corticosteroid fludrocortisone, which is a mineralocorticoid receptor agonist. Fludrocortisone acetate (0.1-5 μg/animal) promotes sodium retention in rats in a dose-dependent manner with 37% of sodium excreted compared with control when used at a dose of 5 μg/animal. Formulations containing fludrocortisone acetate have been used in the treatment of Addison’s disease.
Alflorone Acetate,MSD,US,1954
ChEBI: An acetate ester resulting from the formal condensation of the primary hydroxy group of fludrocortisone with acetic acid. A synthetic corticosteroid, it has glucocorticoid actions about 10 times as potent as hydrocortisone, while its mineralocorticoid acti
ns are over 100 times as potent. It is used in partial replacement therapy for primary and secondary adrenocortical insufficiency in Addison's disease and for the treatment of salt-losing adrenal hyperplasia.
Hydrocortisone acetate is first reacted with phosphorus oxychloride in pyridine
to give the corresponding olefin. Then a sequence consisting of hypobromous
acid addition, ring closure to the epoxide and ring opening with hydrogen
fluoride gives fludrocortisone acetate. Preparation of a crystalline product is
described then in US Patent 2,957,013.
9-Fluoro-11β,17,21-trihydroxy-pregn-4-ene-3,20-dione
acetate
Fludrocortisone acetate,21-acetyloxy-9-fluoro-11β,17-dihydroxypregn-4-ene-3,20-dione, 9α-fluorohydrocortisone (Florinef Acetate), isused only for the treatment of Addison disease and for inhibitionof endogenous adrenocortical secretions. It has up to about 800 times the MC activity of hydrocortisone and about 11 times the GC activity. Its potent activity stimulated the synthesis and study of the many fluorinated steroids. Although its great salt-retaining activity limits its use to Addison disease, it has sufficient GC activity that in some cases of the disease, additional GCs need not be prescribed.
Fludrocortisone acetate is a synthetic corticosteroid with more mineralocorticoid than glucocorticoid activity.
Fludrocortisone acetate is mineralocorticoid. It is used as a replacement therapy in adrenal insufficiency. Fludrocortisone acetate tablets USP, 0.1 mg are indicated as partial replacement therapy for primary and secondary adrenocortical insufficiency in Addison's disease and for the treatment of salt-losing adrenogenital syndrome. ludrocortisone is a man-made form of a natural substance (glucocorticoid) made by the body. It is used along with other medications (such as hydrocortisone) to treat low glucocorticoid levels caused by disease of the adrenal gland (such as Addison's disease, adrenocortical insufficiency, salt-losing adrenogenital syndrome). Glucocorticoids are needed in many ways for the body to function well. They are important for salt and water balance and keeping blood pressure normal. They are also needed to break down carbohydrates in your diet.
Veterinary Drugs and Treatments
Fludrocortisone is used in small animal medicine for the treatment
of adrenocortical
insufficiency (Addison’s disease). It can also be
used as adjunctive therapy in hyperkalemia.
Additionally, in humans, fludrocortisone has been used in saltlosing,
congenital adrenogenital syndrome
and in patients with severe
postural hypotension.
Potentially hazardous interactions with other drugs
Aldesleukin: avoid concomitant use.
Antibacterials: metabolism accelerated by rifamycins;
metabolism possibly inhibited by erythromycin;
possibly reduce isoniazid concentration.
Anticoagulants: efficacy of coumarins and
phenindione may be altered.
Antiepileptics: metabolism accelerated by
carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone.
Antifungals: increased risk of hypokalaemia with
amphotericin - avoid; metabolism possibly inhibited
by itraconazole and ketoconazole.
Antivirals: concentration possibly increased by
ritonavir.
Cobicistat: concentration of fludrocortisone
increased.
Vaccines: high dose corticosteroids can impair
immune response to vaccines - avoid concomitant
use with live vaccines
Fludrocortisone is hydrolysed to produce the non�esterified alcohol. In human volunteers, excretion through
urine was about 80%, and it was concluded that about
20% were excreted by a different route. It is likely that, as
for the metabolism of other steroids, excretion into the
bile is balanced by re-absorption in the intestine and some
part is excreted with the faeces