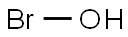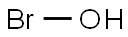Hypobromous acid is a weak, unstable acid with the
chemical formula HBrO, where the bromine atom is in
the +1 oxidation state. It is also called “bromanol” or
“hydroxidobromine”. It occurs only in solution and
has chemical and physical properties that are very
similar to those of hypochlorous acid, HClO. In aqueous
solution, hypobromous acid only partially dissociates
into the hypobromite anion BrO- and the cation, H+.
The salts of hypobromous acid are called hypobromites.
Like the acid, these salts are unstable and when evaporated
or boiled to dryness, they undergo a disproportionation
reaction, yielding the respective bromate and
bromide salts.