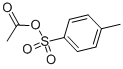
(4-Methylphenyl)sulfonyl acetate prepared from Acetyl Chloride and
p-Toluenesulfonic Acid[1].
the solid is readily hydrolyzed, so it must be protected from contact
with moisture; thermal decomposition is also possible.
The most likely impurity is p-toluenesulfonic acid (could be up to 10%). This can be removed by dissolving it in dry Et2O and cooling until the anhydride crystallises out. It decomposes on heating; below ~130o it gives the disulfonic anhydride and above ~130o polymers are formed, but it can be distilled in a vacuum if it is free of acid. It is used for cleaving ethers [Prep, IR, NMR: Karger & Mazur J Org Chem 36 528, Karger & Mazur J Org Chem 36 532 1971]. [Beilstein 11 III 255.]
[1]. (a) Karger, M. H.; Mazur, Y. JOC 1971, 36, 528. (b) Lin, J. S.; Sleezer, P. D. U.S. Patent 4 219 495, 1980.