Clindamycin, a derivative of lincomycin, was first isolated from Streptomyces lincolnesis in 1962 and became commercially available in 1966. The name originates from Lincoln, Nebraska, where the organism was first isolated from a soil sample. The parent compound of clindamycin is lincomycin, which is produced by actinomycete, Streptomyces liconelnensis, which belongs to the order of Actinobacteria. It replaced lincomycin use because of its better absorption and clinical spectrum. Lincomycin belongs to the lincosamides, which is a class of antibiotics. The compounds use as antibiotic agents stems from their ability to interfere with the protein synthesis of bacteria. The chemical modification of lincomycin, clindamycin, has proven to be superior to the natural product for clinical applications. Clindamycin is better absorbed from the gastrointestinal tract and food does not interfere with its absorption. It is also eight times more active against aerobic gram-positive cocci, and it has proven activity against gram-positive and gram-negative anaerobic bacteria as well as protozoal organisms like Toxoplasma and Plasmodium species.
Dalacin-C,Diethelm,Switz.,1968
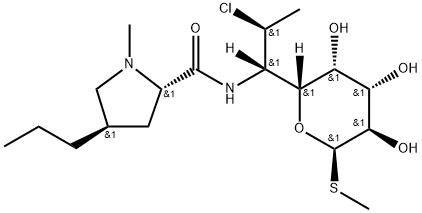
Clindamycin is a semi-synthetic analogue of lincomycin, prepared by chloride substitution of the exocyclic sugar hydroxy group. This affords a more hydrophobic compound with improved pharmacodynamics. Like other members of the lincosamide family, clindamycin is a broad spectrum antibiotic with activity against anaerobic bacteria and protozoans. Clindamycin acts by binding to the 23S ribosomal subunit, blocking protein synthesis. Clindamycin has been extensively studied with over 8,000 literature citations.
ChEBI: Clindamycin is a carbohydrate-containing antibiotic that is the semisynthetic derivative of lincomycin, a natural antibiotic. The physiologic effect of clindamycin is by means of Decreased Sebaceous Gland Activity, and Neuromuscular Blockade.
Clindamycin (Cleocin), 300 to 450 mg/day, is an extremely effective agent for
acne.
Cleocin (Pharmacia &
Upjohn).
Clindamycin is a lincosamide antibiotic. It is active against Gram-positive bacteria, including various strains of S. pneumoniae, S. viridans, S. aureus, and S. epidermidis (MICs = 0.002-0.1, 0.005-0.2, 0.04-1.6, and 0.1-0.2 μg/ml, respectively). Clindamycin is also active against chloroquine-resistant and -sensitive strains of P. falciparum (IC50s = 3.12 and 8.81 nM, respectively). It inhibits bacterial protein synthesis by interacting with the 50S ribosome. Clindamycin increases survival in a mouse model of a secondary S. pneumoniae infection when administered at a dose of 15 mg/kg twice daily for seven days. Formulations containing clindamycin have been used in the treatment of bacterial infections.
This lincosanide antibiotic is used in topical form for acne,
or systemically has been responsible for exanthematous
rashes and acute generalized exanthematous pustulosis.
Clindamycin inhibits bacterial protein synthesis by binding to the 50S ribosomal subunit. The use of clindamycin with macrolides is not recommended since both of them compete for binding sites to the 50S subunit.
Clindamycin is effective in the treatment of most infections secondary to anaerobes and gram-positive cocci. It can be used for anaerobic pulmonary, intra-abdominal, gynecologic, pelvic, diabetic foot, and decubitus ulcer infections. Another appropriate agent should be added since the majority of these infections are polymicrobial. It can also be used as an alternative agent for patients with severe penicillin allergy. It is also used to treat Clostridium perfringens infection.
Oral preparations of clindamycin and vaginal cream are alternatives to metronidazole for the treatment of bacterial vaginosis. Topical solution is used for treatment of acne vulgaris and rosacea.
Clindamycin is extensively metabolized by the liver and the half-life is prolonged in patients with cirrhosis and hepatitis. Dose reductions are recommended in patients with acute liver disease.
The most commonly observed adverse effect is diarrhea. The reported incidence of C. difficile colitis in patients treated with clindamycin varies from 0.1 to 10%. The syndrome may be fatal. If the patient develops C. difficile colitis, clindamycin should be discontinued and the patient should be treated for C. difficile . Other side effects include rash, nausea, vomiting, diarrhea, flatulence, abdominal distension, anorexia, and transient elevation of liver enzymes. Other less common events, such as fever, neutropenia, thrombocytopenia, and eosinophilia have been reported.
Clindamycin, methyl-[7-chloro-6,7,8-trideoxy-6-trans-(1-methyl-4-propyl�L-2-pyrrollidin-carboxamido)-1-thio-L-threo-α-D-galacto-octapyranoside] (32.5.2), which
is a 7(S)-chloro-7-deoxy derivative of lincomycin, is synthesized by replacing the hydroxyl
group of lincomycin (32.5.1) at C7 by treating it with triphenyl phophine in acetonitrile
(Raydon reagent), in which a configuration transformation takes place in the given carbo hydrate.
Veterinary Drugs and Treatments
Topical clindamycin is an optional topical treatment for feline acne.
Clindamycin inhibits bacterial protein synthesis by binding to the 50S ribosome; primary activity is against anaerobic and grampositive
aerobic bacteria. For more information on the pharmacology of clindamycin, refer to the monograph for systemic use found in
the main section.
Potentially hazardous interactions with other drugs
Ciclosporin: may cause reduced ciclosporin levels.
Erythromycin: antagonism demonstrated in vitro;
manufacturers recommend that the two drugs should
not be administered concurrently.
Muscle relaxants: enhanced neuromuscular blockade.
Clindamycin undergoes metabolism, presumably in the
liver, to the active N-demethyl and sulfoxide metabolites,
and also to some inactive metabolites. About 10% of a
dose is excreted in the urine as active drug or metabolites
and about 4% in the faeces; the remainder is excreted as
inactive metabolites. Excretion is slow, and takes place
over several days