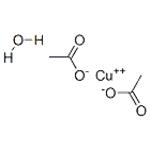
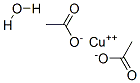Bluish-green fine powder; hygroscopic. The monohydrate is dimeric; density 1.88 g/cm3; melts at 115°C; decomposes at 240°C; soluble in water and ethanol; and slightly soluble in ether.
Copper(II) acetate is used as a pigment for ceramics; in the manufacture of Paris green; in textile dyeing; as a fungicide; and as a catalyst.
Copper(II) acetate monohydrate is used in DNA extraction in biochemical applications. It is also used as a catalyst or oxidizing agent in organic syntheses.
Catalyst for activation of alcohols as greener alkylating reagents.
Copper-Catalyzed Aerobic Oxidation of Amines to Imines under Neat Conditions with Low Catalyst Loading.
Copper(II) acetate is prepared by treatment of copper(II) oxide, CuO, or copper(II) carbonate, CuCO3, with acetic acid, followed by crystallization:
CuO + 2CH3COOH → (CH3COO)2Cu + H2O
Copper(II) acetate is moderately toxic by ingestion and possibly other routes of administration.
LD50 oral (rat): c. 600 mg/kg
Copper acetate monohydrate is a greenish Blue powder or small crystals with odour of acetic acid.
Copper(II) acetate monohydrate is used in formation of Cu chelates of ?-dicarbonyl compounds as a means of purification and in the eglinton (modified glaser) coupling of terminal alkynes. It is also used as a catalyst for the michael reaction giving increased yields and absence of side-reactions. It Promotes ullmann-type C-O and C-N coupling reactions of arylboronic acids with phenols, amines and various other nitrogen derivatives. It is also used in biochemical applications such as DNA extraction.
Copper(II) acetate monohydrate can easily be produced by the reaction of acetic acid solutions with copper(II) carbonate or copper(II) hydroxide or by the reaction of copper(II) oxide with hot acetic acid. Large-scale commercial production uses copper metal in the presence of air and refluxing acetic acid.
Copper(II) acetate monohydrate is used as a catalyst in the polymerization of organic materials and as a mordant in the dying of textiles. It is also used as a ceramic pigment and as a fungicide.
Copper (II) acetate monohydrate is soluble in water and slightly soluble in methanol, diethylether and acetone. It can be synthesized by reacting acetic acid with copper (II) carbonate or copper(II) hydroxide or copper (II) oxide. Large scale commercial production is undertaken by placing copper metal in the presence of air and refluxing acetic acid. As a transition metal acetate, it can be used to form oxide nanoparticles by sonochemical methods.
reagent type: catalyst
core: copper
Cupric acetate is used as a fungicide, as a catalyst for organic reactions; in textile dyeing and as a pigment for ceramics.
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Recystallise it twice from warm dilute acetic acid solutions (5mL/g) by cooling. [Beilstein 2 IV 111.]
Forms explosive materials with acetylene gas, ammonia, caustic solutions; sodium hypobromite; notromethane. Keep away from chemically active metals; strong acids; nitrates. Decomposes above 240C forming acetic acid fumes
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill.