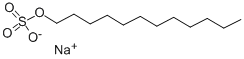
Dodecyl Sulfate Sodium appears as white to pale yellow paste or liquid with a mild odor. Sinks and mixes with water.
Sodium dodecyl sulfate is non-toxic, slightly soluble in alcohol, insoluble in chloroform and ether, soluble in water, and has good anionic and nonionic complex compatibility. It has good emulsibility, foamability, and foaming, infiltrating, decontaminating and dispersing properties.It is abundant in foams and quickly biodegradable, and has solubility next only to fatty alcohol polyoxyethylene ether sodium sulphate (abbreviated as AES). It is not sensitive to alkali and hard water, but its stability is inferior to general sulfonate under acidic conditions and is close to AES.
Dodecyl sulfate sodium is an emulsifier and whipping aid that has a solubility of 1 g in 10 ml of water. It functions as an emulsifier in egg whites. It is used as a whipping aid in marshmallows and angel food cake mix. It also functions to aid in dissolving fumaric acid.
Inhalation of dust causes sneezing and coughing. Ingestion of large amounts causes irritation of stomach. Dust irritates eyes and may cause burns on prolonged contact. Contact with skin causes some irritation; continued exposure to water solution causes drying out and cracking.
Pharmaceutical Applications
Dodecyl Sulfate Sodium is an anionic surfactant employed in a wide range of nonparenteral pharmaceutical formulations and cosmetics.It is a detergent and wetting agent effective in both alkaline and acidic conditions.
Dodecyl Sulfate Sodium helps to quickly disrupt the biological membranes.It is usually used as one of the major constituent of several reagents, that are used to purify nucleic acids.SDS can block the activity of RNase and deoxyribonuclease (DNase).
Dodecyl Sulfate Sodium can be synthesized by reacting dodecyl alcohol with sulfur trioxide gas, followed by neutralization with sodium hydroxide. The preparation of SDS involves the following steps: The reaction takes place in a vertical reactor at 32 °C. Nitrogen gas is introduced through the gas vents at a flow rate of 85.9 L/min. Lauryl alcohol is added at a flow rate of 58 g/min at 82.7 kPa. Liquid sulfur trioxide is fed into the flash evaporator at 124.1 kPa, with a flow rate of 0.9072 kg/h and a flash temperature of 100 °C. The sulfated product is quickly cooled to 50 °C, aged for 10-20 min, then neutralized with a base in a neutralization kettle controlled at 50 °C. The pH is adjusted to 7-8.5, and the liquid product is spray dried to obtain a solid product.
Toxicity LD50 is 1300mg/kg. There is no evidence that this product is carcinogenic, but high doses may indeed irritate the skin. However, in general sanitary products the concentration is limited when used as a forming agent, and is in line with national standards.
Dodecyl Sulfate Sodium reacts with cationic surfactants, causing loss of activity even in concentrations too low to cause precipitation. Unlike soaps, it is compatible with dilute acids and calcium and magnesium ions.
Dodecyl Sulfate Sodium is incompatible with salts of polyvalent metal ions, such as aluminum, lead, tin or zinc, and precipitates with potassium salts. Solutions of dodecyl sulfate sodium (pH 9.5–10.0) are mildly corrosive to mild steel, copper, brass, bronze, and aluminum.