Sodium dodecyl sulfate: Application, preparation and toxicity
General description
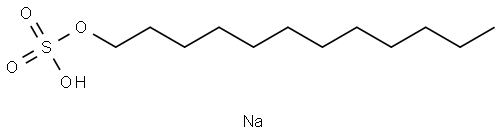
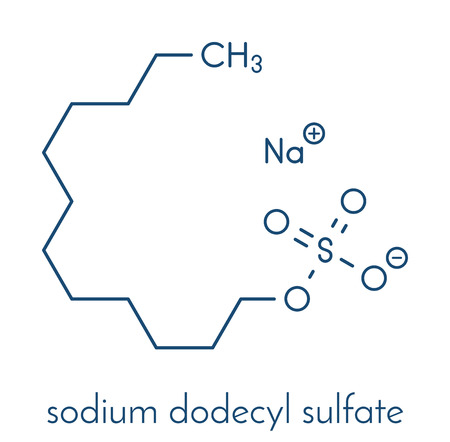
Sodium dodecyl sulfate (SDS) is the sodium salt ester of lauryl alcohol (1-dodecanol) and is officially designated sulfuric acid monododecyl ester sodium salt. It is an anionic surfactant used in many cleaning and hygiene products. This molecule is an organosulfate and a salt. It consists of a 12-carbon tail attached to a sulfate group, that is, it is the sodium salt of dodecyl hydrogen sulfate, the ester of dodecyl alcohol and sulfuric acid. Its hydrocarbon tail combined with a polar "headgroup" give the compound amphiphilic properties and so make it useful as a detergent. Also derived as a component of mixtures produced from inexpensive coconut and palm oils, Sodium dodecyl sulfate is a common component of many domestic cleaning, personal hygiene and cosmetic, pharmaceutical, and food products, as well as of industrial and commercial cleaning and product formulations. Its appearance is as follows:

Figure 1 Appearance of Sodium dodecyl sulfate.
Application
The surface-active nature of SOS makes it very useful in a multitude of diverse applications: as an ingredient in many consumer products, as an aid in manufacturing processes, and as a biological research tool. All of these take advantage of its ability to solubilize fats and oils, lower the surface tension of aqueous solutions, or form microemulsions. SOS is used extensively in the cosmetics industry as a detergent, wetting, foaming, and emulsifying agent and is among the most frequently used anionic surfactants in shampoos. Many uses of Sodium dodecyl sulfate in biological and biomedical research stem from its ability to solubilize lipid membranes. Its cytolytic properties are very useful in making preparations of subcellular materials for biochemical studies, and it has been utilized for protein identification and structure determination by polyacrylamide gel electrophoresis (SDS-PAGE). Sodium dodecyl sulfate is commonly used in manufacturing, and its ability to form microemulsions by lowering surface tension at liquid-solid interfaces is widely used in the electroplating industry, particularly with nickel and zinc [1].
Preparation
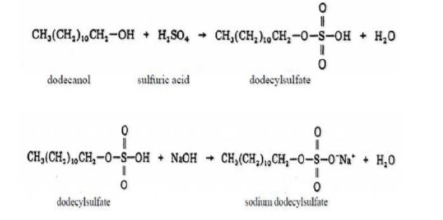
Sodium dodecyl sulfate is synthesized by treating lauryl alcohol with sulfur trioxide gas, oleum, or chlorosulfuric acid to produce hydrogen lauryl sulfate [2]. The resulting product is then neutralized through the addition of sodium hydroxide or sodium carbonate. Lauryl alcohol can be used in pure form or may be derived from either coconut or palm kernel oil by hydrolysis (which liberates their fatty acids), followed by hydrogenation. When produced from these sources, commercial samples of these "SDS" products are actually not pure Sodium dodecyl sulfate, rather a mixture of various sodium alkyl sulfates with SDS being the main component [3]. For instance, Sodium dodecyl sulfate is a component, along with other chain-length amphiphiles, when produced from coconut oil, and is known as sodium coco sulfate (SCS). Sodium dodecyl sulfate is available commercially in powder, pellet, and other forms (each differing in rates of dissolution), as well as in aqueous solutions of varying concentrations.
Toxicity
Sodium dodecyl sulfate enhances the skin penetration of some chemicals. Trans- epidermal delivery of systemic-action drugs has become increasingly attractive over oral delivery because it avoids variable absorption and first-pass metabolism, allows for continuous drug input and the use of drugs with short half-lives, facilitates the rapid termination of a drug's effect, and fosters improved patient compliance [4]. Chowhan and Pritchard (1978) found that Sodium dodecyl sulfate increased in vitro naproxen flux appreciably and that differences in species and surfactant concentration had marked effects on absorption enhancement [5]. Use of isopropanol as a vehicle doubled the penetration enhancement properties of SDS in human skin in vitro. Sodium dodecyl sulfate is not carcinogenic when consumed or applied directly, even to amounts and concentrations that exceed amounts used in standard commercial products [6].The earlier review of the Cosmetic Ingredient Review (CIR) program Expert Panel in 1983 reported that Sodium dodecyl sulfate (there, abbreviated SLS, for sodium lauryl sulfate) in concentrations up to 2%, in a year-long oral dietary studies in dogs, gave no evidence of tumorigenicity or carcinogenicity, and that no excess chromosomal aberrations or clastogenic effects were observed in rats fed up to 1.13% sodium lauryl sulfate in their diets for 90 days, over those on a control diet.
References
[1]Kirsner JB, Wolff RA (1944) The effect of sodium alkyl sulfates on the peptic activity of the gastric contents and on the healing of gastric ulcer in man. Gastro-enterology 2:93-101.
[2]Takei, Kensuke; Tsuto, Keiichi; Miyamoto, Shigeyuki; Wakatsuki, Junya (1985). Anionic surfactants: lauric products. Journal of the American Oil Chemists' Society. 62 (2): 341–347.
[3]Gloxhuber, C., & Kunster, K. (1992). Anionic Surfactants: Biochemistry, toxicology, dermatology (2nd ed.). New York.
[4]Kastner W (1992) Pharmacological properties. In: Gloxhuber Ch, Kiinstler K (eds) Anionic surfactants: Biochemistry, toxicology, dermatology, 2nd ed. Marcel Dekker, New York, pp 419-448.
[5]Chowhan ZT, Pritchard R (1978) Effect of surfactants on percutaneous absorption of naproxen. I. Comparison of rabbit, rat, and human excised skin. J Pharmacol Sci 67(9):1272-1274.
[6]Cosmetic Ingredient Review (CIR) program Expert Panel (1983). Final Report on the Safety Assessment of Sodium Lauryl Sulfate and Ammonium Lauryl Sulfate. Int. J. Toxicol. 2 (7): 127–81.
Related articles And Qustion
See also
Lastest Price from Sodium dodecyl sulfate manufacturers

US $100.00/kg2025-11-14
- CAS:
- 151-21-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons

US $1200.00-1100.00/ton2025-11-03
- CAS:
- 151-21-3
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M