dye
Dyes refer to the organic substances enable the strong coloring of fiber and some other materials. There are various kinds of dyes. According to the source, they can be divided into natural dyes (such as vegetable dyes, animal dyes and mineral dyes, etc.) and synthetic dyes (or artificial dyes). According to the molecular structure, it can be divided into azo dyes, anthraquinone dyes, phthalocyanine dyes, aromatic dyes and nitro dyes. Based on the application methods, it can be divided into acid dyes, basic dyes, sulfur dyes, reactive dyes, disperse dyes, direct dyes and so on. Dyes have color, but substance having color does not necessarily belong to dyes. Dye must have a chromophore and auxochrome group. Dye contained in the ink should also have water-soluble group such as sulfonic acid group.
Natural dyes are dyes obtained from animals, plants and minerals. According to sources they can be divided into:
1 Plant dyes; dyes extracted from the roots, stems, leaves and fruit of some plants such as indigo extracted from the leaves of indigo (blue); curcumin extracted from turmeric (yellow); alizarin extracted from madder (red) and so on;
2 Animal dyes; dyes extracted from animal body such as carmine extracted from cochineal, etc;
3 Mineral dyes; dyes extracted from the colored inorganic substance of mineral such as chrome yellow, ultramarine blue, brown and other manganese. Owing to its various disadvantages compared with artificial dyes such as incomplete chromatography, application inconvenience and poor fastness, most natural dyes have been eliminated except for a few still in use.
Synthetic dyes, also known as "artificial dyes." is mainly made through the chemical processing of coal tar (or oil processing) fractionation products (such as benzene, naphthalene, anthracene and carbazole, etc.), sometimes are also known as coal tar dyes. Since the earliest several synthetic dyes all took aniline as raw material, it is also known as "aniline dyes." Compared with the natural dyes, synthetic dyes have various kinds with complete chromatography and most of them being colorful, washable and being able to be produced in large scale. Thus, almost all of the current so-called dye refers to synthetic dyes with its dyeing products being one of the common samples of Forensic evidence.
There are two ways for classifications of dyes:
The first type is the basic chemical taxonomy based on the chromophore. According to this, dyes can be classified into azo dyes, sulfur dyes, anthraquinone dyes, indigo dyes, heterocyclic dyes and phthalocyanine dyes, 10 categories in total.
The second type is based on the property of the dye to the fiber or coloring properties. Based on this, the dyes can be divided into acid dyes, neutral dyes, azoic dyes, basic dyes, cationic dyes, direct dyes, reactive dyes, sulfur dyes, vat dyes and other dyes, 14 categories in total.
There are various kinds of dyes of different properties and wide range of applications. However, dyes used as physical evidence are largely fixed in textile fibers or paper, or as a kind of organizational component of ink, printing ink and pen oil. Usually after extraction, it can subject to comparison testing using micro-chemical method, thin layer chromatography, liquid chromatography, UV - visible absorption spectroscopy and fluorescence spectroscopy.
The international development of dye was very rapid in 1970s while people have mainly focused on develop new varieties and improve quality since 1990s with the annual output being maintained at the level of 100 million tons.
Chinese dyes got rapid development in 1980s with the main focus being improving the quality after 1990 as well. The production output still maintain a certain growth rate with annual output being around 300,000 t. In 2000, the output is 315 600 t. Dyes used for textile dyeing account about 85% with the rest being used for the coloring of leather, plastic, paper and paint.
- Structure:
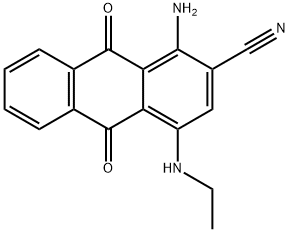
- Chemical Name:Disperse Blue 359
- CAS:62570-50-7
- MF:C17H13N3O2
- Structure:
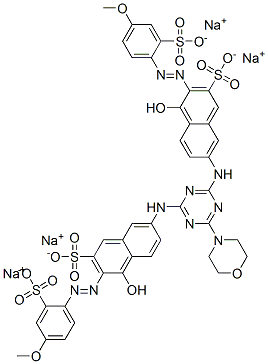
- Chemical Name:tetrasodium 7,7'-[[6-(morpholin-4-yl)-1,3,5-triazine-2,4-diyl]diimino]bis[4-hydroxy-3-[(4-methoxy-2-sulphonatophenyl)azo]naphthalene-2-sulphonate]
- CAS:2184-11-4
- MF:C41H32N10Na4O17S4
- Structure:
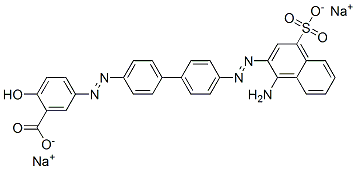
- Chemical Name:disodium 5-[[4'-[(1-amino-4-sulphonato-2-naphthyl)azo][1,1'-biphenyl]-4-yl]azo]salicylate
- CAS:2429-79-0
- MF:C29H19N5Na2O6S
- Structure:
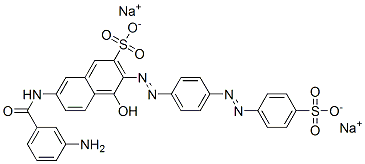
- Chemical Name:disodium 7-[(3-aminobenzoyl)amino]-4-hydroxy-3-[[4-[(4-sulphonatophenyl)azo]phenyl]azo]naphthalene-2-sulphonate
- CAS:5979-31-7
- MF:C29H20N6Na2O8S2
- Chemical Name:Direct Blue 201
- CAS:60800-55-7
- MF:C30H16Cl2N4Na2O8S2
- Chemical Name:Direct Blending Blue D-RGL
- CAS:
- MF:C42H25O13N7S4Na4
- Chemical Name:Disperse Black Crude for Sublimation Ink
- CAS:
- MF:
- Structure:
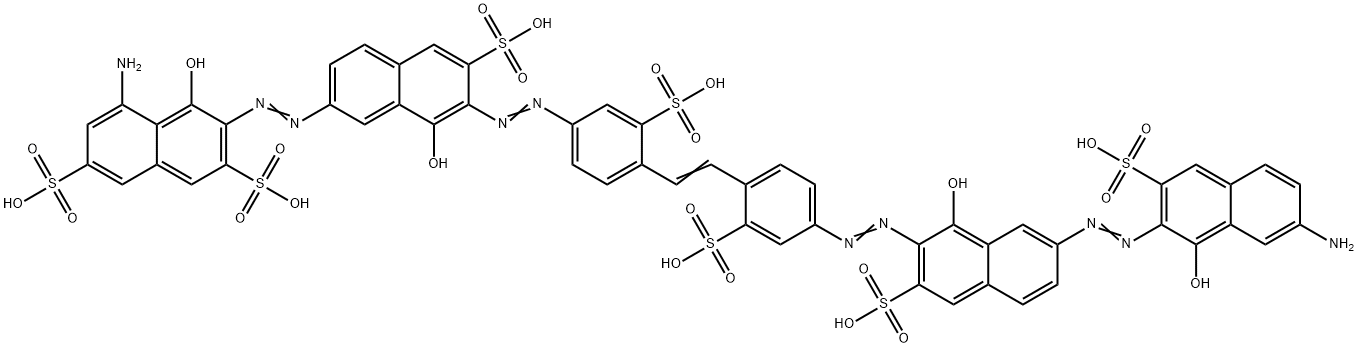
- Chemical Name:Direct Blending Navy Blue D-R
- CAS:72586-15-3
- MF:C54H38N10O25S7
- Chemical Name:DIRECT BLACK 22
- CAS:
- MF:
- Structure:
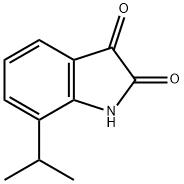
- Chemical Name:7-ISOPROPYLISATIN
- CAS:57816-97-4
- MF:C11H11NO2
- Chemical Name:DIRECT BROWN 210
- CAS:
- MF:
- Structure:
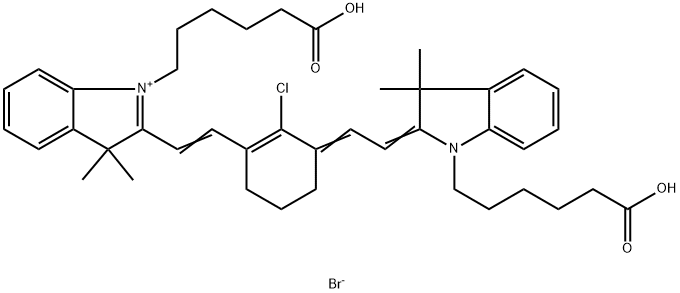
- Chemical Name:MHI-148
- CAS:172971-76-5
- MF:C42H52BrClN2O4
- Chemical Name:DIRECT BROWN 210
- CAS:12222-29-6
- MF:
- Chemical Name:ACID BLUE 281
- CAS:
- MF:
- Structure:
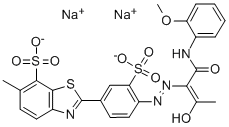
- Chemical Name:DIRECT YELLOW 27
- CAS:10190-68-8
- MF:C25H20N4Na2O9S3
- Chemical Name:Tartrazine
- CAS:477549-79-4
- MF:
- Structure:
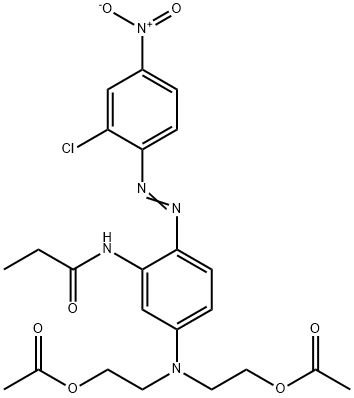
- Chemical Name:Disperse Red S-2GFL
- CAS:26850-12-4
- MF:C23H26ClN5O7
- Structure:
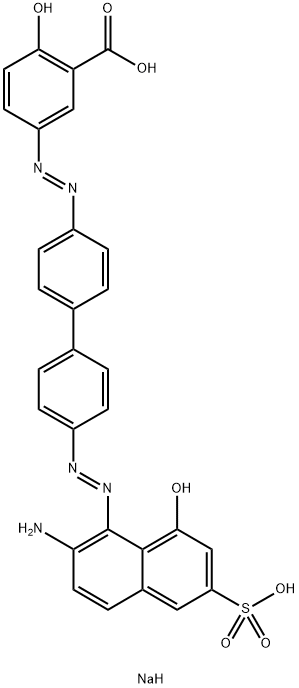
- Chemical Name:disodium 5-[[4'-[(2-amino-8-hydroxy-6-sulphonato-1-naphthyl)azo][1,1'-biphenyl]-4-yl]azo]salicylate
- CAS:2429-84-7
- MF:C29H22N5NaO7S
- Structure:
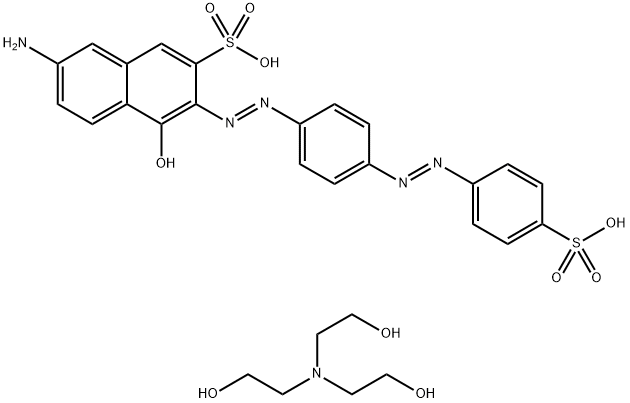
- Chemical Name:7-amino-4-hydroxy-3-[[4-[(4-sulphophenyl)azo]phenyl]azo]naphthalene-2-sulphonic acid, compound with 2,2',2''-nitrilotriethanol (1:2)
- CAS:64683-40-5
- MF:C28H32N6O10S2
- Chemical Name:Direct Red 239
- CAS:60202-35-9
- MF:
- Structure:
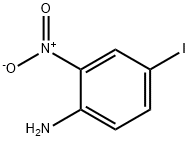
- Chemical Name:4-Iodo-2-nitroaniline
- CAS:20691-72-9
- MF:C6H5IN2O2
- Structure:

- Chemical Name:disodium 7-[[3-[(4-aminobenzoyl)amino]benzoyl]amino]-4-hydroxy-3-[[4-[(4-sulphonatophenyl)azo]phenyl]azo]naphthalene-2-sulphonate
- CAS:5905-22-6
- MF:C36H25N7Na2O9S2
- Structure:
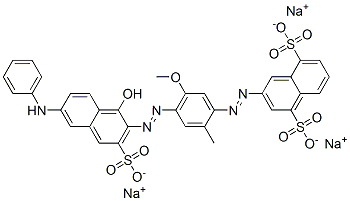
- Chemical Name:trisodium 3-[[4-[[6-(anilino)-1-hydroxy-3-sulphonato-2-naphthyl]azo]-5-methoxy-o-tolyl]azo]naphthalene-1,5-disulphonate
- CAS:6227-20-9
- MF:C34H24N5Na3O11S3
- Structure:
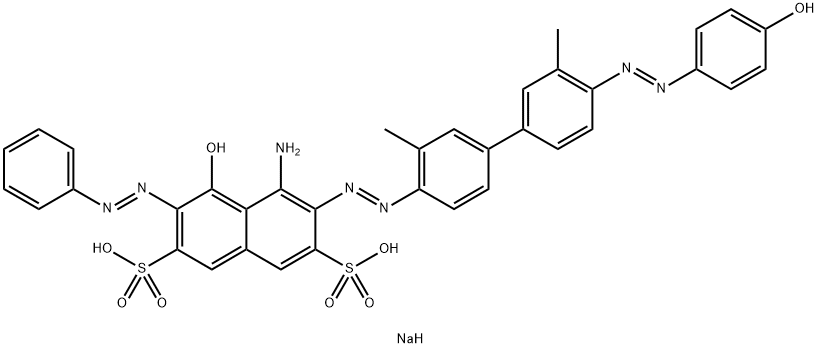
- Chemical Name:Direct Green 85
- CAS:72390-60-4
- MF:C36H30N7NaO8S2
- Chemical Name:Direct Blending Brilliant Yellow D-GL
- CAS:
- MF:
- Structure:
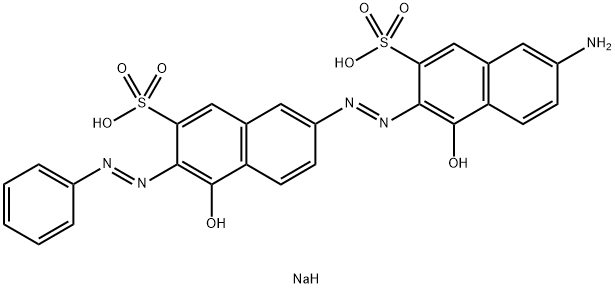
- Chemical Name:Direct Red 16
- CAS:6227-02-7
- MF:C26H20N5NaO8S2
- Structure:
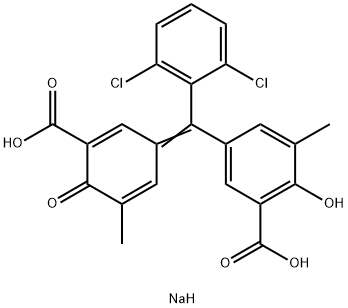
- Chemical Name:Chromeazurol B
- CAS:1796-92-5
- MF:C23H17Cl2NaO6
- Chemical Name:Neutral red indicator
- CAS:
- MF:
- Chemical Name:DIRECT SCARLET 4BS
- CAS:
- MF:
- Structure:
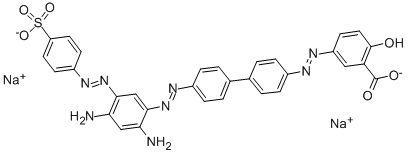
- Chemical Name:DIANIL BROWN 3GN
- CAS:3811-71-0
- MF:C31H22N8Na2O6S
- Structure:

- Chemical Name:disodium 3-[[4-[[4-[(2,4-diamino-5-nitrophenyl)azo]-m-tolyl]azo]-o-tolyl]azo]naphthalene-1,5-disulphonate
- CAS:6739-48-6
- MF:C30H26N9NaO8S2
- Chemical Name:DIRECT BLUE 200
- CAS:12217-57-1
- MF:
- Chemical Name:Direct Blending Yellow D-3RNL
- CAS:
- MF:C68H48O26N16S8Na8
- Structure:
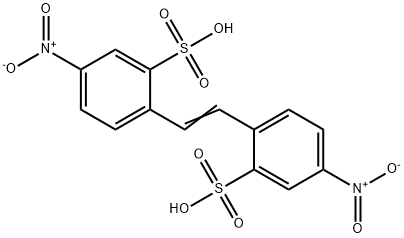
- Chemical Name:4,4'-Dinitrostilbene-2,2'-disulfonic acid
- CAS:128-42-7
- MF:C14H10N2O10S2
- Structure:
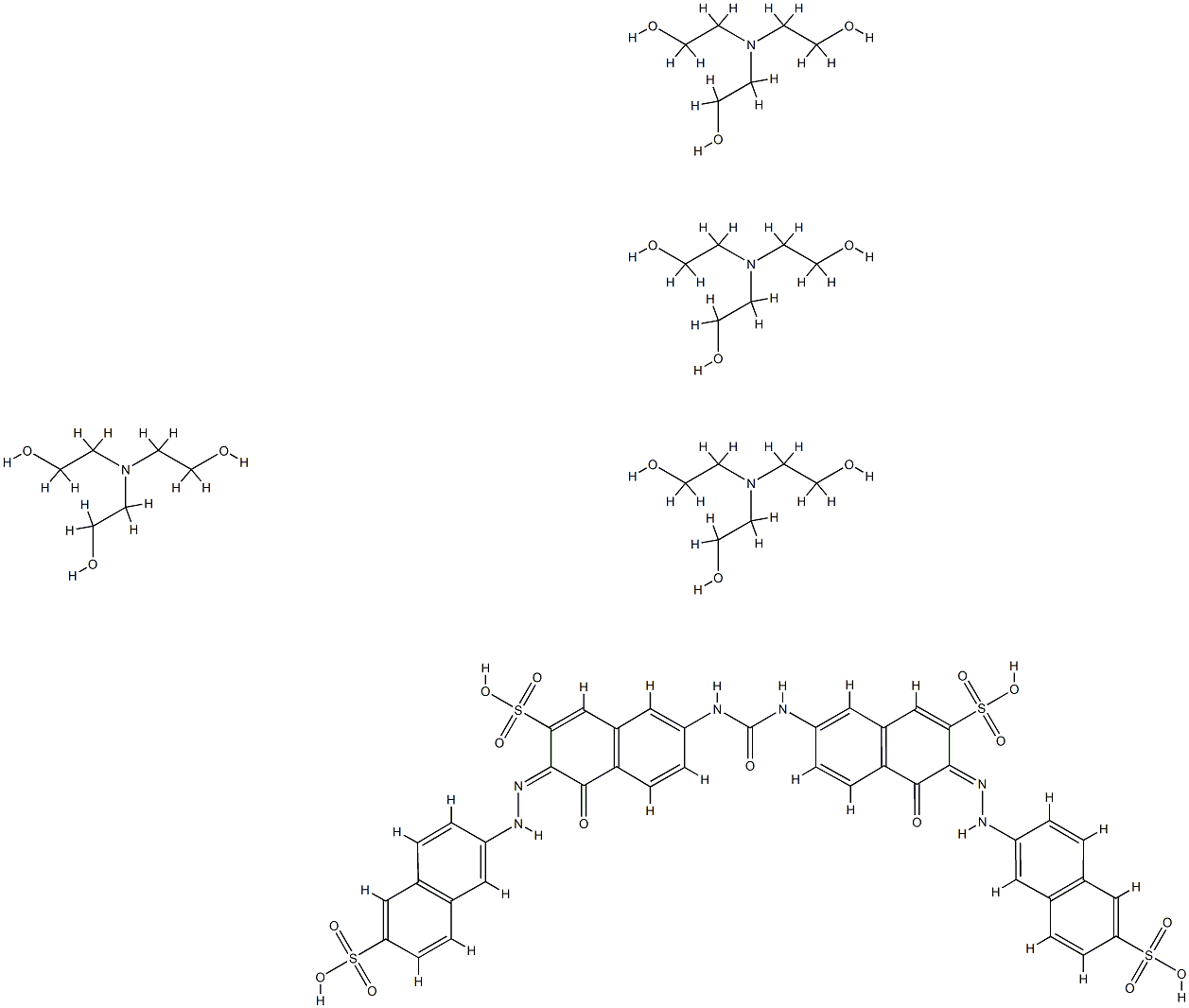
- Chemical Name:7,7'-(carbonyldiimino)bis[4-hydroxy-3-[(6-sulpho-2-naphthyl)azo]naphthalene-2-sulphonic] acid, compound with 2,2',2''-nitrilotriethanol (1:4)
- CAS:36596-36-8
- MF:C65H88N10O27S4
- Structure:
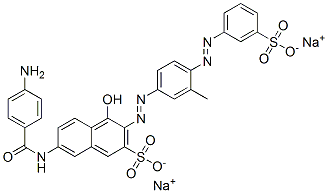
- Chemical Name:disodium 7-[(4-aminobenzoyl)amino]-4-hydroxy-3-[[3-methyl-4-[(3-sulphonatophenyl)azo]phenyl]azo]naphthalene-2-sulphonate
- CAS:5979-32-8
- MF:C30H22N6Na2O8S2
- Structure:
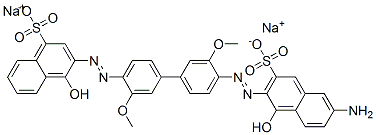
- Chemical Name:disodium 3-[[4'-[[6-amino-1-hydroxy-3-sulphonato-2-naphthyl]azo]-3,3'-dimethoxy[1,1'-biphenyl]-4-yl]azo]-4-hydroxynaphthalene-1-sulphonate
- CAS:3818-60-8
- MF:C34H25N5Na2O10S2
- Structure:
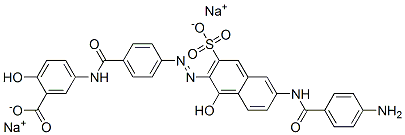
- Chemical Name:disodium 5-[[4-[[6-[(4-aminobenzoyl)amino]-1-hydroxy-3-sulphonato-2-naphthyl]azo]benzoyl]amino]salicylate
- CAS:6771-94-4
- MF:C31H21N5Na2O9S
- Structure:
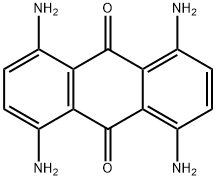
- Chemical Name:DISPERSE BLUE 1
- CAS:2475-45-8
- MF:C14H12N4O2
- Structure:
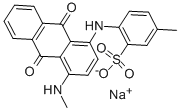
- Chemical Name:ALIZARIN ASTROL
- CAS:6408-51-1
- MF:C22H17N2NaO5S
- Structure:
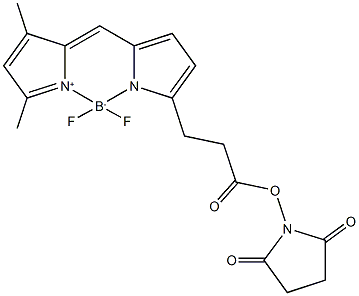
- Chemical Name:BDP FL NHS ester
- CAS:146616-66-2
- MF:C18H18BF2N3O4
- Structure:
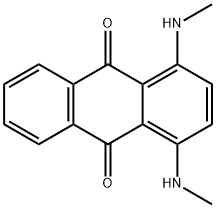
- Chemical Name:Disperse Blue 14
- CAS:2475-44-7
- MF:C16H14N2O2
- Structure:
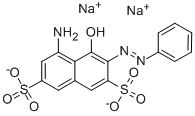
- Chemical Name:AZOFUCHSIN
- CAS:3567-66-6
- MF:C16H11N3Na2O7S2
- Chemical Name:Direct fasting
- CAS:
- MF:
- Chemical Name:Acid Red 361
- CAS:61931-22-4
- MF:
- Structure:
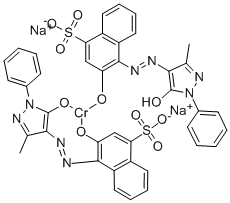
- Chemical Name:Acid violet 90
- CAS:6408-29-3
- MF:C40H27CrN8O10S2.2Na
- Structure:
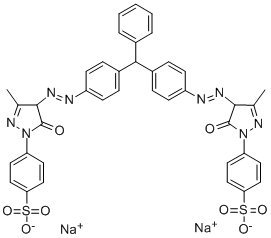
- Chemical Name:Acid Yellow 117
- CAS:6459-70-7
- MF:C39H30N8O8S2.2Na
- Structure:

- Chemical Name:Acid Brown 75
- CAS:8011-86-7
- MF:C10H9NO7S2
- Structure:
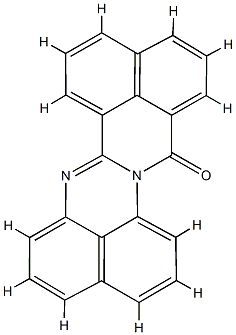
- Chemical Name:Solvent Red 179
- CAS:89106-94-5
- MF:C22H12N2O
- Structure:
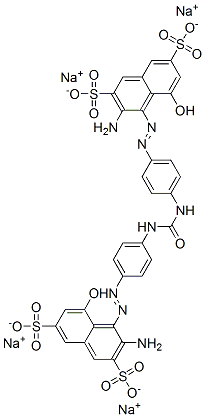
- Chemical Name:tetrasodium 4,4'-[carbonylbis(imino-4,1-phenyleneazo)]bis[3-amino-5-hydroxynaphthalene-2,7-disulphonate]
- CAS:6771-81-9
- MF:C33H22N8Na4O15S4
- Structure:
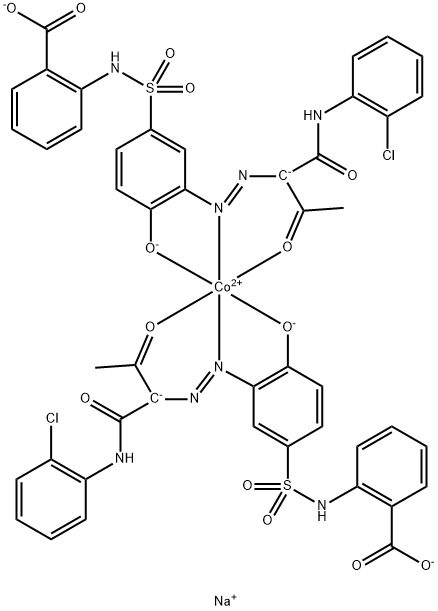
- Chemical Name:Acid Yellow 220
- CAS:70851-34-2
- MF:C46H32Cl2CoN8NaO14S2(-3)
- Structure:
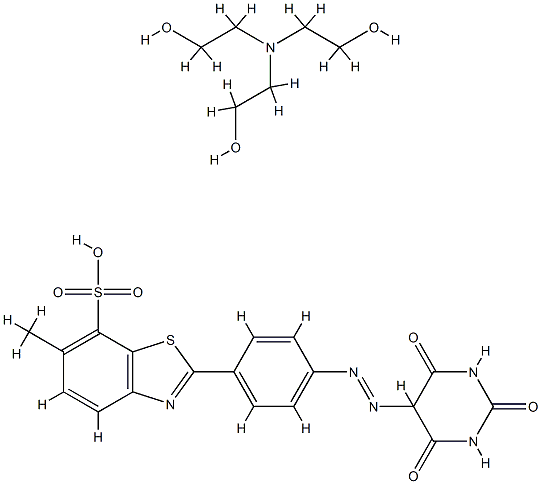
- Chemical Name:2-[4-[(hexahydro-2,4,6-trioxo-5-pyrimidyl)azo]phenyl]-6-methylbenzothiazole-7-sulphonic acid, compound with 2,2',2''-nitrilotris[ethanol] (1:1)
- CAS:65036-46-6
- MF:C24H28N6O9S2
- Chemical Name:Direct Red 224
- CAS:12222-48-9
- MF:
- Chemical Name:Solvent Blue 3R
- CAS:
- MF:
- Structure:
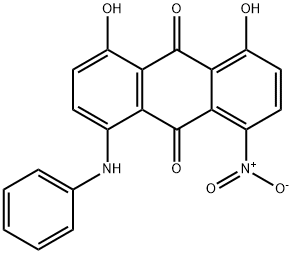
- Chemical Name:Disperse Blue 77
- CAS:20241-76-3
- MF:C20H12N2O6
- Chemical Name:Acid Yellow 116
- CAS:12239-18-8
- MF:C217H15IN4O5S
- Chemical Name:Basic Yellow 29
- CAS:39279-59-9
- MF:C13H17ClN6O2
- Structure:
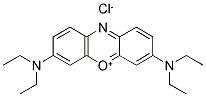
- Chemical Name:Phenazoxonium,3,7-bis(diethylamino)-,nitrate
- CAS:73570-52-2
- MF:C20H26N3O.NO3
- Structure:
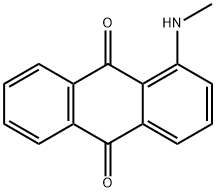
- Chemical Name:Disperse Red 9
- CAS:82-38-2
- MF:C15H11NO2
- Structure:
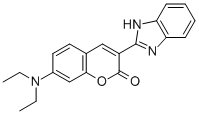
- Chemical Name:DISPERSE YELLOW 82
- CAS:12239-58-6
- MF:C20H19N3O2
- Structure:
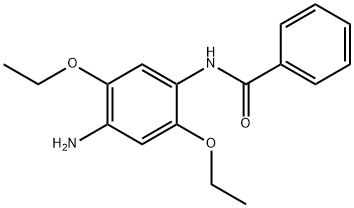
- Chemical Name:Fast Blue BB
- CAS:120-00-3
- MF:C17H20N2O3
- Structure:
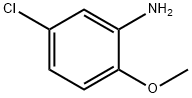
- Chemical Name:5-Chloro-2-methoxyaniline
- CAS:95-03-4
- MF:C7H8ClNO
- Chemical Name:Acid Red 131
- CAS:12234-99-0
- MF:
- Structure:
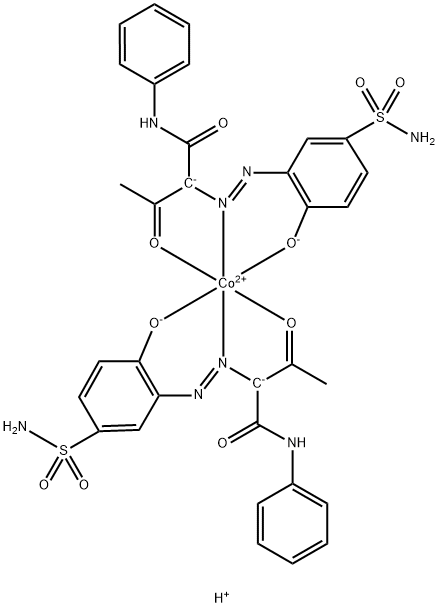
- Chemical Name:Acid Yellow 4R
- CAS:12715-61-6
- MF:C32H29CoN8O10S2-
- Structure:
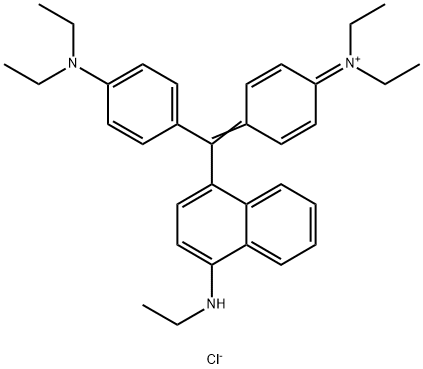
- Chemical Name:Basic Blue 7
- CAS:2390-60-5
- MF:C33H40ClN3
- Structure:
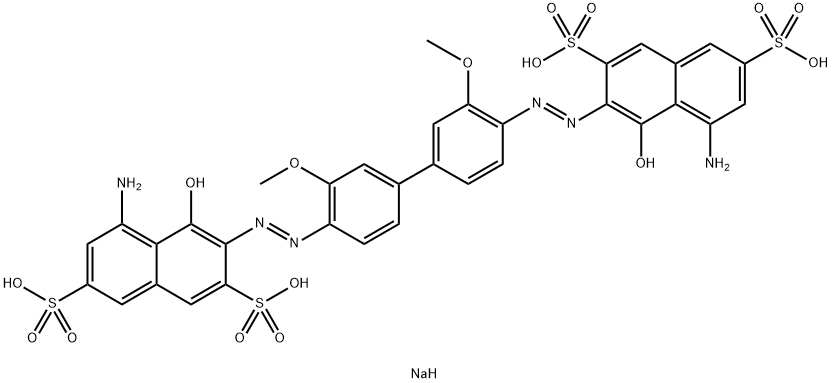
- Chemical Name:DIRECT BLUE 15
- CAS:2429-74-5
- MF:C34H29N6NaO16S4
- Structure:
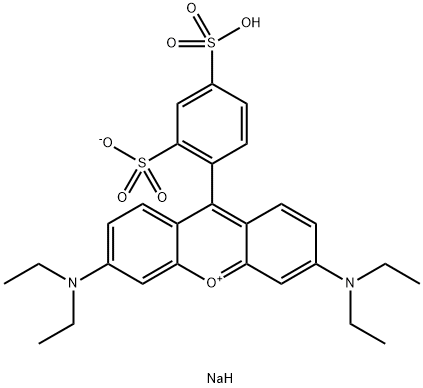
- Chemical Name:Sulforhodamine B
- CAS:3520-42-1
- MF:C27H31N2NaO7S2
- Structure:
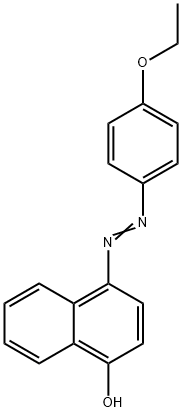
- Chemical Name:Solvent Red 3
- CAS:6535-42-8
- MF:C18H16N2O2
- Structure:

- Chemical Name:EOSIN Y
- CAS:
- MF:C20H8Br4O5
- Structure:
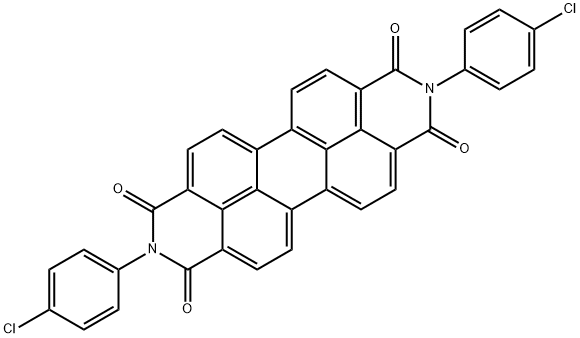
- Chemical Name:Vat Red 32
- CAS:2379-77-3
- MF:C36H16Cl2N2O4
- Chemical Name:Solvent Blue 45
- CAS:37229-23-5
- MF:
- Chemical Name:Solvent Violet 37
- CAS:61969-50-4
- MF:
- Chemical Name:Solvent Blue 70
- CAS:12237-24-0
- MF:
- Structure:
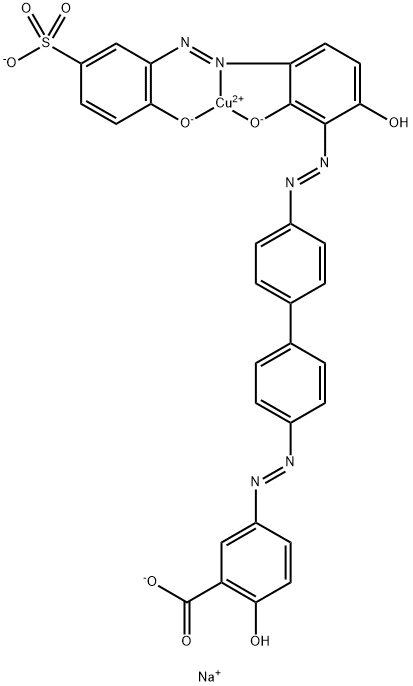
- Chemical Name:Direct Brown 95
- CAS:16071-86-6
- MF:C31H18CuN6NaO9S-
- Chemical Name:Acid Yellow 219
- CAS:71819-57-3
- MF:C20H17N4NaO5S
- Structure:
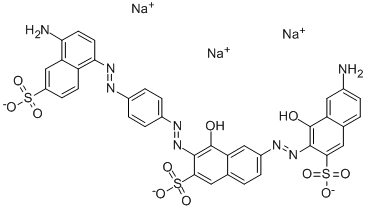
- Chemical Name:Direct Black 80
- CAS:8003-69-8
- MF:C36H23N8O11S3.3Na
- Chemical Name:Acid Black 172
- CAS:61847-77-6
- MF:C40H24N6O14S2.Cr.2Na
- Structure:
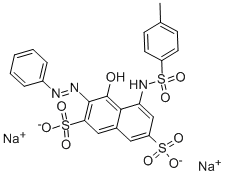
- Chemical Name:ACID RED 106
- CAS:6844-74-2
- MF:C23H17N3Na2O9S3
- Structure:
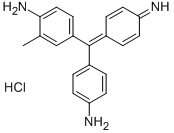
- Chemical Name:FUCHSIN BASIC
- CAS:632-99-5
- MF:C20H20ClN3
- Structure:
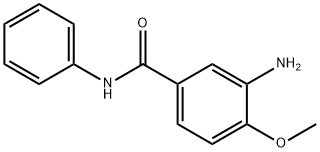
- Chemical Name:3-Amino-4-methoxybenzanilide
- CAS:120-35-4
- MF:C14H14N2O2
- Structure:
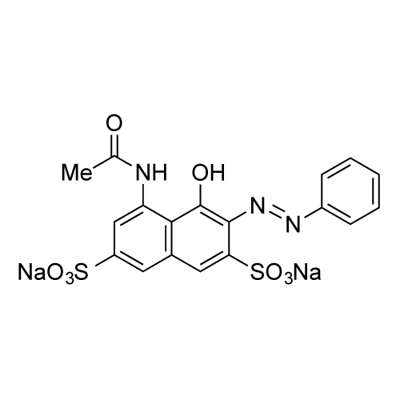
- Chemical Name:ACID RED 1
- CAS:3734-67-6
- MF:C18H13N3Na2O8S2
- Structure:
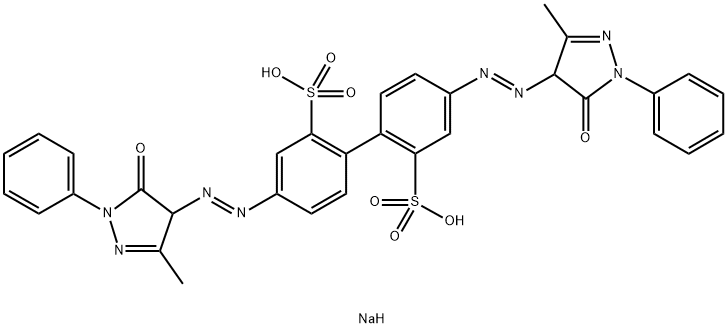
- Chemical Name:Acid Yellow 42
- CAS:6375-55-9
- MF:C32H27N8NaO8S2
- Structure:

- Chemical Name:Solvent Blue 5
- CAS:1325-86-6
- MF:C33H41N3O
- Structure:
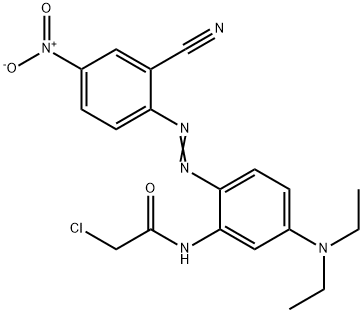
- Chemical Name:Disperse Violet 63
- CAS:64294-88-8
- MF:C19H19ClN6O3
- Structure:
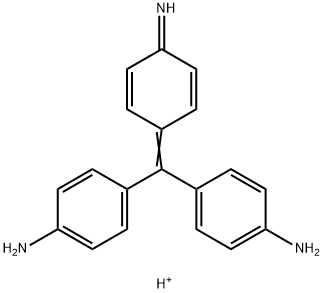
- Chemical Name:CI 42500
- CAS:25620-78-4
- MF:C19H18N3+
- Structure:
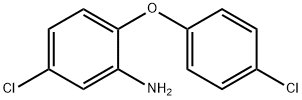
- Chemical Name:2-AMINO-4,4'-DICHLORODIPHENYL ETHER
- CAS:121-27-7
- MF:C12H9Cl2NO
- Structure:
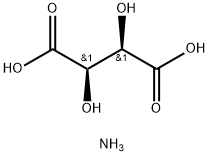
- Chemical Name:AMMONIUM HYDROGEN TARTRATE
- CAS:3095-65-6
- MF:C4H9NO6
- Structure:
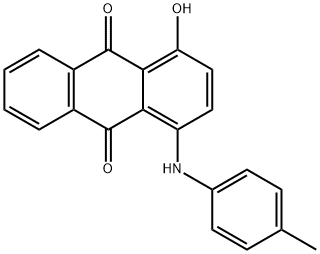
- Chemical Name:Solvent Violet 13
- CAS:81-48-1
- MF:C21H15NO3
- Structure:
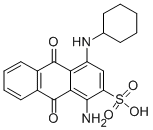
- Chemical Name:ACID BLUE 62
- CAS:4368-56-3
- MF:C20H20N2O5S
- Structure:
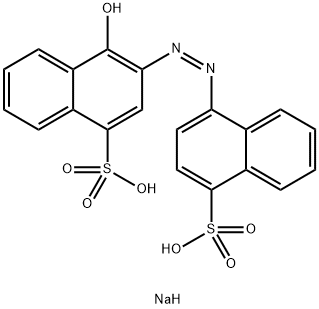
- Chemical Name:C.I. 14720
- CAS:3567-69-9
- MF:C20H15N2NaO7S2
- Structure:
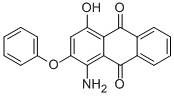
- Chemical Name:DISPERSE RED 60
- CAS:12223-37-9
- MF:C20H13NO4
- Structure:
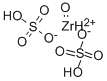
- Chemical Name:Zirconium oxide sulfate
- CAS:62010-10-0
- MF:O5SZr
- Structure:
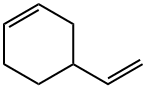
- Chemical Name:4-Vinyl-1-cyclohexene
- CAS:100-40-3
- MF:C8H12
- Chemical Name:Acid Brown 349
- CAS:72827-73-7
- MF:
- Chemical Name:Acid Brown 165
- CAS:61724-14-9
- MF:
- Structure:
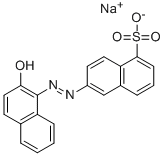
- Chemical Name:ACID RED 9
- CAS:8003-59-6
- MF:C20H13N2NaO4S
- Structure:
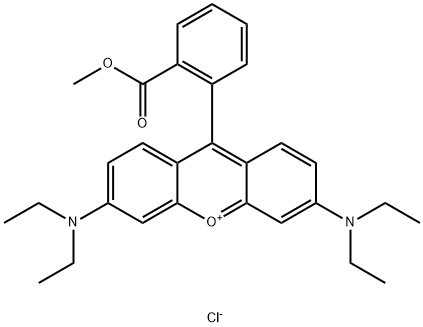
- Chemical Name:CRYSTAL VIOLET
- CAS:39393-39-0
- MF:C29H33ClN2O3
- Structure:
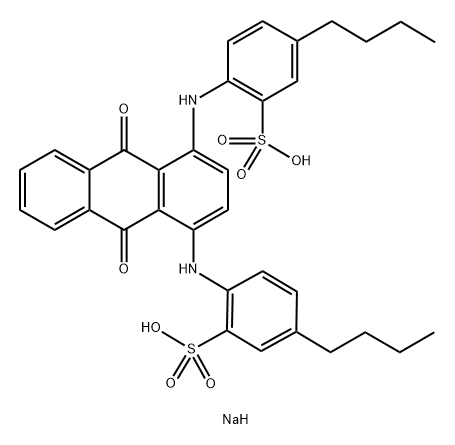
- Chemical Name:ACID GREEN 27 (C.I. 61580)
- CAS:6408-57-7
- MF:C34H35N2NaO8S2
- Chemical Name:Acid Black 2
- CAS:8005-03-6
- MF:NULL
- Structure:
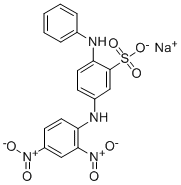
- Chemical Name:Acid Orange 3
- CAS:6373-74-6
- MF:C18H13N4NaO7S