Cationic dyes
Cationic dyes are dyes that can be dissociated into positively charged ions in the aqueous solution. They can interact with the negative group on the fiber molecules to form a salt, further firmly being attached to the fiber so that the fiber is dyed. Cationic dye is a kind of alkaline dye and is successfully developed on the fundamental of the alkaline dyes. However, different from the stained principle of the alkaline dyes, the cationic dye dyes the fiber through the binding of its cation ion to the acidic group in the third monomer with of the acrylon, thus leading to high fastness, but can easily lead to uneven dyeing. Therefore, dyeing retarding agent is often added to improve the dyeing properties. Cationic dye is the dedicated dye for dying the acrylic fiber. In addition, it can also be used to modify the dyeing and printing of the polyester and nylon. It is mainly used for dyeing the polyacrylonitrile fibers. So far, the general old varieties are still called alkaline dyes with the new varieties called as cationic dyes.
There are many types of cationic dyes such as azo dyes, triarylmethane dyes, anthraquinone dyes, and heterocyclic compounds. The commonly used is the cationic portion in the dyes having an onium group to form salt with hydrochloric acid (or sulfuric acid). At this time, most of the cations are N + ions. Such dyes are water-soluble with stronger affinity and high fastness than acrylon. But on other hand, it is not easy to migrate once infected, resulting in poor leveling. For this reason, retarding agent may be added. There are two ways for the connection between coloring conjugated systems of the cationic dyes with onium group. The first one belongs to conjugated type. Such dyes have bright color, strong coloring capability and have the advantage of alkaline dyes, For example: methine dye, tris diphenylmethane dyes and azomethine dyes. The second is the isolated version with the conjugated system being connected with onium group via isolation group (i.e. positive charge is attached to the nitrogen atom of the quaternary ammonium salt). This class of dye has poorer color brightness and coloring power compared with conjugated type, but has excellent light resistance and heat resistance. It is mostly applied to light-colored dying. For example: anthraquinone dyes, azo dyes.
Because of the strong binding of such dyes due to the interaction of its cation affinity with the introduced fiber anionic groups, the dying has high fastness. However, the portion with high dying concentration is difficult to migrate to the part of low dye concentration, thus easily resulting in uneven dyeing defects.
Cationic dye is now mostly applied to the dyeing of the polyester fiber and acrylic fiber. If you introduce an acidic group to the di-acetate cellulose and vinyl chloride fiber, it can also be used for dyeing. For some kinds of blended fibered products, when adopting a cationic dye, we can choose carrier dyeing or high temperature dyeing. For some blends (e.g., polyester fiber and wool fiber), we uses a combination of a cationic dye dyes, acid dyes, direct dyes, reactive dyes and so on. In this case, there will be phenomenon of precipitation and tarring. At this time, we can adopt anti precipitant. It has recently been developed of such kinds of accommodated such a dispersion-type cationic dye blend, namely being manufactured from the combination between cationic dye and phosphomolybdic acid, certain complex or the combination between the condensation substance obtained from naphthalenesulfonic acid with formaldehyde and lignin sulfonic acids, etc. For this kind of dye, if the temperature starts to rise, the cation will be slowly dissociated, being able to be used in combination with other kinds of cationic acids such as acid dyes, direct dyes and reactive dyes.
There are many types of cationic dyes with varied manufacturing processes as well. For example: the manufacturing process of methine dyes and azomethine dyes is via the condensation reaction between aromatic aldehyde with the active methylene and aromatic amines or the condensation reaction between aromatic diazonium salts with active methylene. Azomethine dye is usually manufactured through the azo coupling reaction. Triphenylmethane dyes is synthesized through the condensation between aromatic aldehyde and aromatic amine. Triazine dye is made through the condensation between aromatic nitroso compound and amino phenol, however usually forming hydrochloride and sulfate.
- Structure:
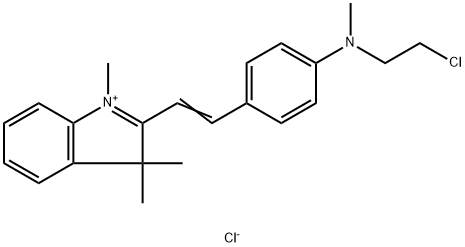
- Chemical Name:ASTRAZON PINK FG
- CAS:3648-36-0
- MF:C22H26Cl2N2
- Structure:
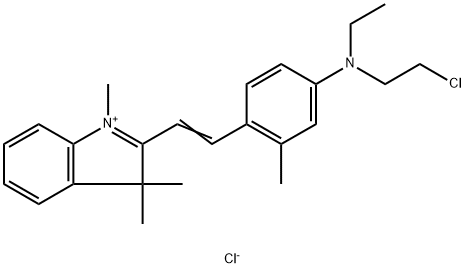
- Chemical Name:ASTRAZON RED 6B
- CAS:6441-82-3
- MF:C24H30Cl2N2
- Structure:
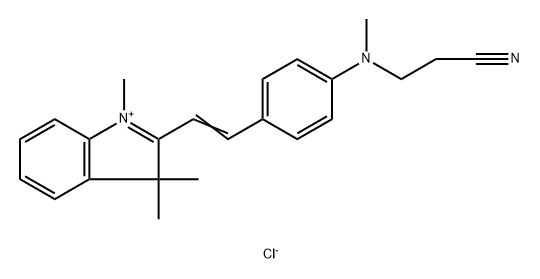
- Chemical Name:ASTRAZON BRILLIANT RED 4G
- CAS:12217-48-0
- MF:C23H26ClN3
- Structure:
- Chemical Name:BASIC YELLOW 11
- CAS:4208-80-4
- MF:C21H25ClN2O2
- Chemical Name:Catonic Orange G
- CAS:
- MF:
- Chemical Name:Catonic Black O
- CAS:
- MF:
- Structure:
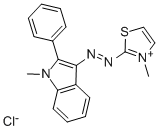
- Chemical Name:BASIC RED 29
- CAS:42373-04-6
- MF:C19H17ClN4S
- Structure:
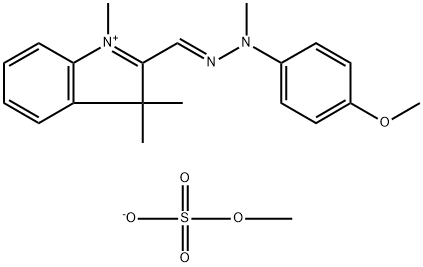
- Chemical Name:BASIC YELLOW 28
- CAS:54060-92-3
- MF:C21H27N3O5S
- Chemical Name:Catonic Yellow X-8GL
- CAS:12217-50-4
- MF:C20H23ON2Cl
- Chemical Name:Basic Blue 159
- CAS:105953-73-9
- MF:
- Chemical Name:Brilliant Red B
- CAS:
- MF:
- Structure:
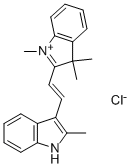
- Chemical Name:BASIC ORANGE 21
- CAS:3056-93-7
- MF:C22H23ClN2
- Structure:
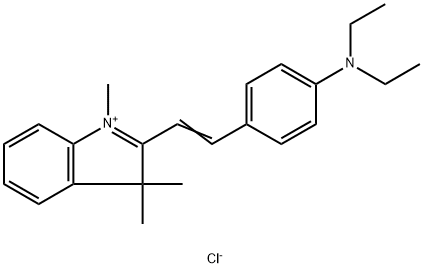
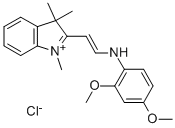
- Chemical Name:2-[2-[4-(diethylamino)phenyl]vinyl]-1,3,3-trimethyl-3H-indolium chloride
- CAS:6359-45-1
- MF:C23H29ClN2
- Chemical Name:CATIONIC DYES
- CAS:
- MF:
- Chemical Name:Cationic Red B
- CAS:
- MF:C25H29Cl2N3
- Chemical Name:Cationic Black X-O
- CAS:
- MF:
- Chemical Name:Cationic Blue GL
- CAS:
- MF:
- Chemical Name:Turquoise Blue X-GB
- CAS:
- MF:
- Chemical Name:Black X-FBL
- CAS:
- MF:
- Chemical Name:Cationic starch
- CAS:
- MF:
- Structure:
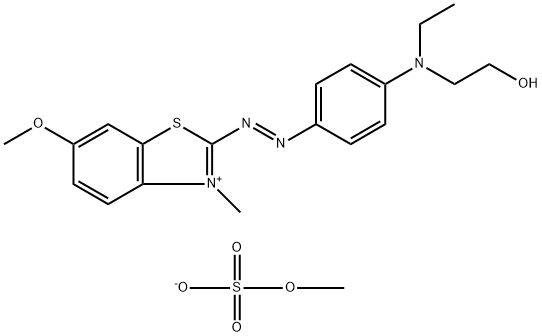
- Chemical Name:BASIC BLUE 41 (C.I. 11105)
- CAS:12270-13-2
- MF:C20H26N4O6S2
- Chemical Name:BASIC BLUE 4
- CAS:55840-82-9
- MF:C20H26N3O.ZnCl3
- Chemical Name:Catonic Black XL
- CAS:
- MF:
- Chemical Name:BASIC RED 18:1
- CAS:12271-12-4
- MF:
- Chemical Name:Cationic Red 6B
- CAS:
- MF:
- Structure:
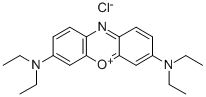
- Chemical Name:BASIC BLUE 3
- CAS:33203-82-6
- MF:C20H26ClN3O
- Structure:
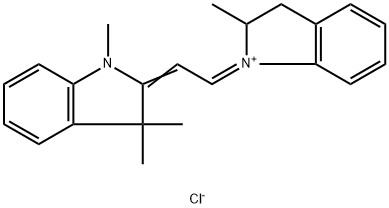
- Chemical Name:Basic Yellow 21
- CAS:6359-50-8
- MF:C22H25ClN2
- Chemical Name:Cationic Red x-GRL
- CAS:
- MF:C18H23N6Cl2Zn
- Structure:
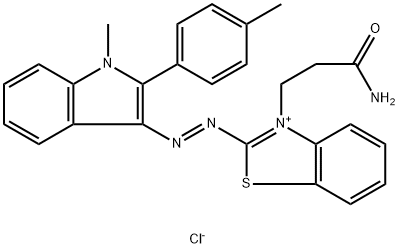
- Chemical Name:3-(3-amino-3-oxopropyl)-2-[[1-methyl-2-(p-tolyl)-1H-indol-3-yl]azo]benzothiazolium chloride
- CAS:12221-63-5
- MF:C26H24ClN5OS
- Chemical Name:Catonic Black L
- CAS:
- MF:
- Chemical Name:Flavine 10GFF
- CAS:
- MF:
- Chemical Name:CATIONIC DYESTUFF
- CAS:
- MF: