basic dyes
Basic dye, also known as basic group dyes, is the salt generated by aromatic bases reacting with acids (organic and inorganic acids), videlicet that is colored organic base salts whose basic group is generally amino, which becomes -NH2 • HCl salt groups when the salt occurs. The groups dissolve in water, dissociated into dye cations and acid anions. Thus the dye is also known as cationic dyes.
In 1856 H.W.perkin synthesized mauveine guilty basic dye, the first synthetic dye in the world. Following then it gradually emerged types of Basic Fuchsin (CI Basic Violet 14), basic blue (CI Basic Blue 9), crystal violet (CI Basic Violet 3), malachite green (CI Basic Green 4) and Rhodamine (CI basic violet 10). The chemical structure of basic dyes includes aromatic methane, triarylmethane, azo type and nitrogen-containing heterocyclic compounds (such as xanthene, oxazine and thiazine, etc.).
There are less hydrophilic groups in basic dyes so that they are insoluble in water. When we dissolve them, we use alcohol or acetic acid and then dilute with water. Basic dyes are sensitive to temperature, so that both temperature of the dissolution-dilution and dye-bath should not be too high. Basic dyes have affinity with leather surface with a negative charge so it is suitable to dye vegetable tanned leather. Mainly used for leather belt anions (vegetable tanned leather) for dyeing, its binding force is strong. It is also used for chrome tanned leather with cationic because it owns light permanency and is seldom used.
Basic dyes are organic alkali salts and when in solution, they dissociate into cations and anions pigment, so they are also known as basic group dyes. The molecular structure generally contains primary amines, secondary amines, tertiary amines or nitrogen-containing heterocyclic ring, leading to the weakly cationic in acid bath.
Basic dye tints strongly with bright shade. But the light permanency and endurance washing natures of such dyes are poor. Thus it is now rarely used for coloring fibers and mainly used for coloring paper, ribbon and biological materials and so on. Crystal Violet, rhodamine and oxazine dyes are also used as thermal dyes, sensitive dyes and dye lasers.
- Structure:
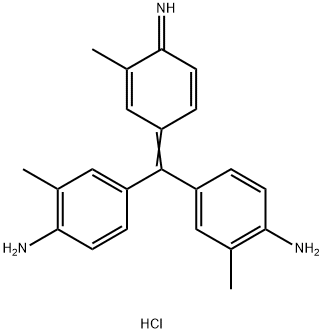
- Chemical Name:Basic Violet 2
- CAS:3248-91-7
- MF:C22H24ClN3
- Structure:
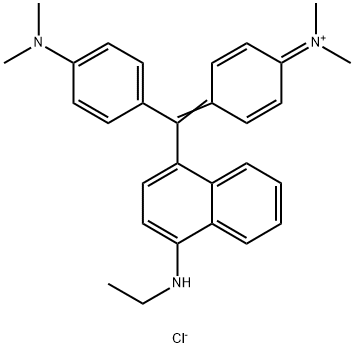
- Chemical Name:Basic Blue 11
- CAS:2185-86-6
- MF:C29H32ClN3
- Structure:
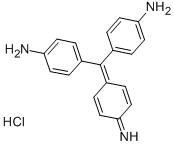
- Chemical Name:Basic Red 9
- CAS:569-61-9
- MF:C19H17N3.ClH
- Structure:
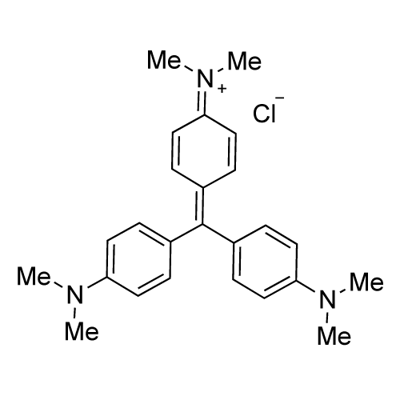
- Chemical Name:Crystal Violet
- CAS:548-62-9
- MF:C25H30ClN3
- Structure:
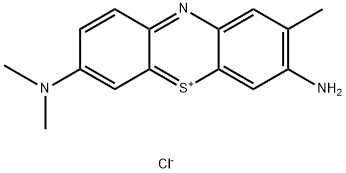
- Chemical Name:Toluidine Blue O
- CAS:92-31-9
- MF:C15H16ClN3S
- Structure:
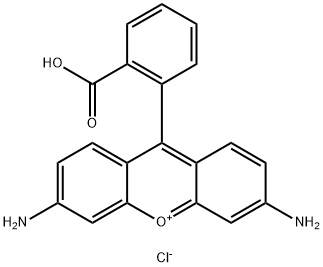
- Chemical Name:RHODAMINE 110
- CAS:13558-31-1
- MF:C20H15ClN2O3
- Structure:
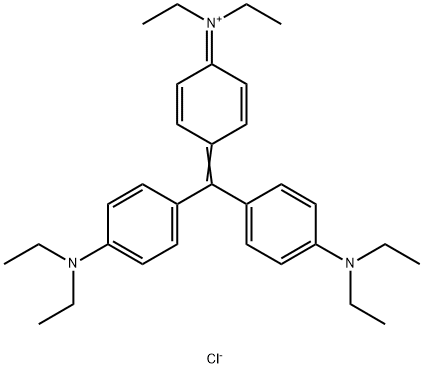
- Chemical Name:ETHYL VIOLET
- CAS:2390-59-2
- MF:C31H42ClN3
- Structure:
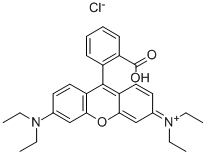
- Chemical Name:Rhodamine B
- CAS:81-88-9
- MF:C28H31ClN2O3
- Structure:
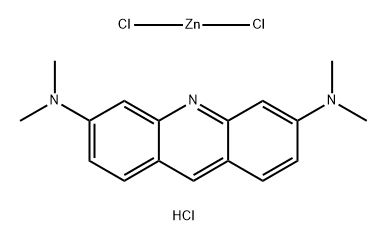
- Chemical Name:Basic Orange 14
- CAS:10127-02-3
- MF:C17H20Cl3N3Zn
- Structure:
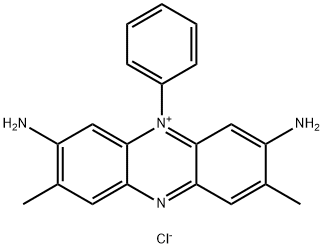
- Chemical Name:Basic Red 2
- CAS:477-73-6
- MF:C20H19ClN4
- Structure:
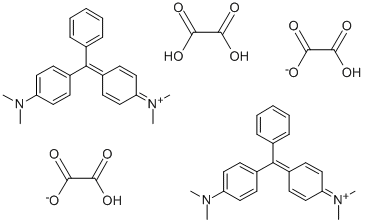
- Chemical Name:Basic Green 4
- CAS:2437-29-8
- MF:C52H54N4O12
- Structure:
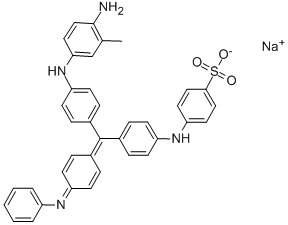
- Chemical Name:ACID BLUE 119
- CAS:30586-13-1
- MF:C32H29N3O4S.Na
- Structure:
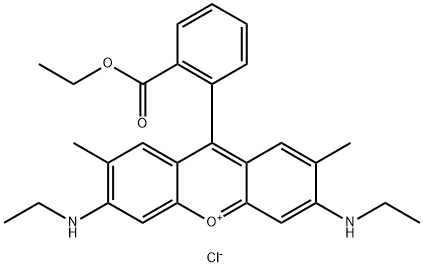
- Chemical Name:Rhodamine 6G
- CAS:989-38-8
- MF:C28H31ClN2O3
- Structure:
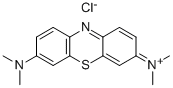
- Chemical Name:Methylene Blue
- CAS:61-73-4
- MF:C16H18ClN3S
- Structure:
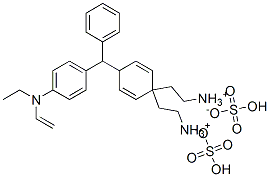
- Chemical Name:Basic Green 1
- CAS:633-03-4
- MF:C27H34N2O4S
- Structure:
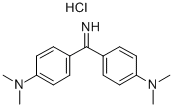
- Chemical Name:AURAMINE O
- CAS:2465-27-2
- MF:C17H22ClN3
- Structure:
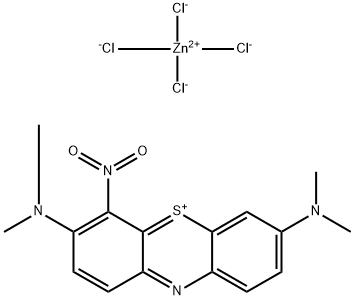
- Chemical Name:Basic Green 5
- CAS:224967-52-6
- MF:C16H17Cl4N4O2SZn-
- Structure:
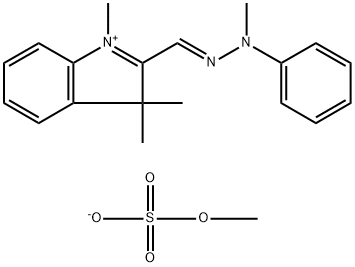
- Chemical Name:Basic Yellow 51
- CAS:83949-75-1
- MF:C20H25N3O4S
- Structure:
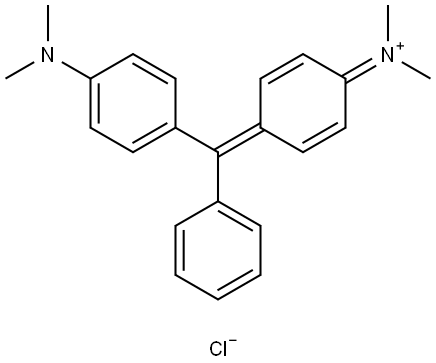
- Chemical Name:Basic Green 4
- CAS:569-64-2
- MF:C23H25N2.Cl
- Structure:
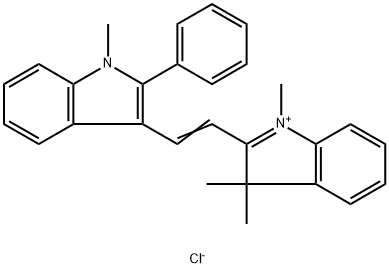
- Chemical Name:ASTRAZON ORANGE R
- CAS:4657-00-5
- MF:C28H27ClN2
- Structure:
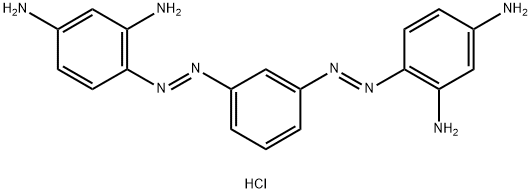
- Chemical Name:Basic Brown 1
- CAS:10114-58-6
- MF:C18H19ClN8
- Structure:
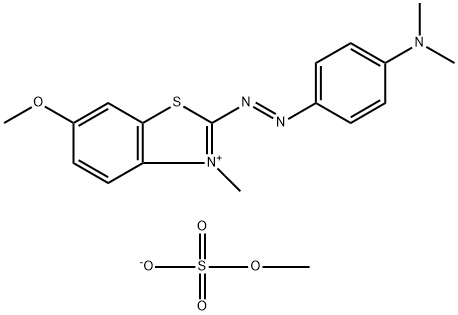
- Chemical Name:Basic Blue 54
- CAS:15000-59-6
- MF:C18H22N4O5S2
- Structure:
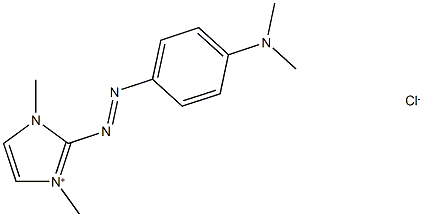
- Chemical Name:Basic Red 51
- CAS:12270-25-6
- MF:C13H18ClN5
- Structure:
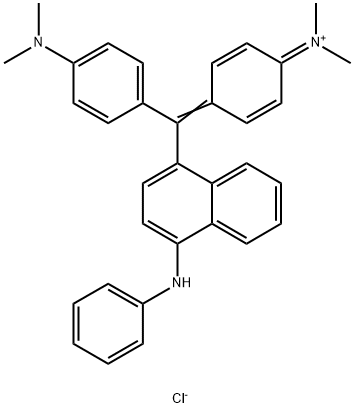
- Chemical Name:Basic Blue 26
- CAS:2580-56-5
- MF:C33H32ClN3
- Structure:
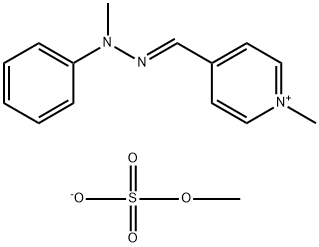
- Chemical Name:methyl 1-methyl-4-[(methylphenylhydrazono)methyl]pyridinium sulphate
- CAS:68259-00-7
- MF:C14H16N3.CH3O4S
- Structure:
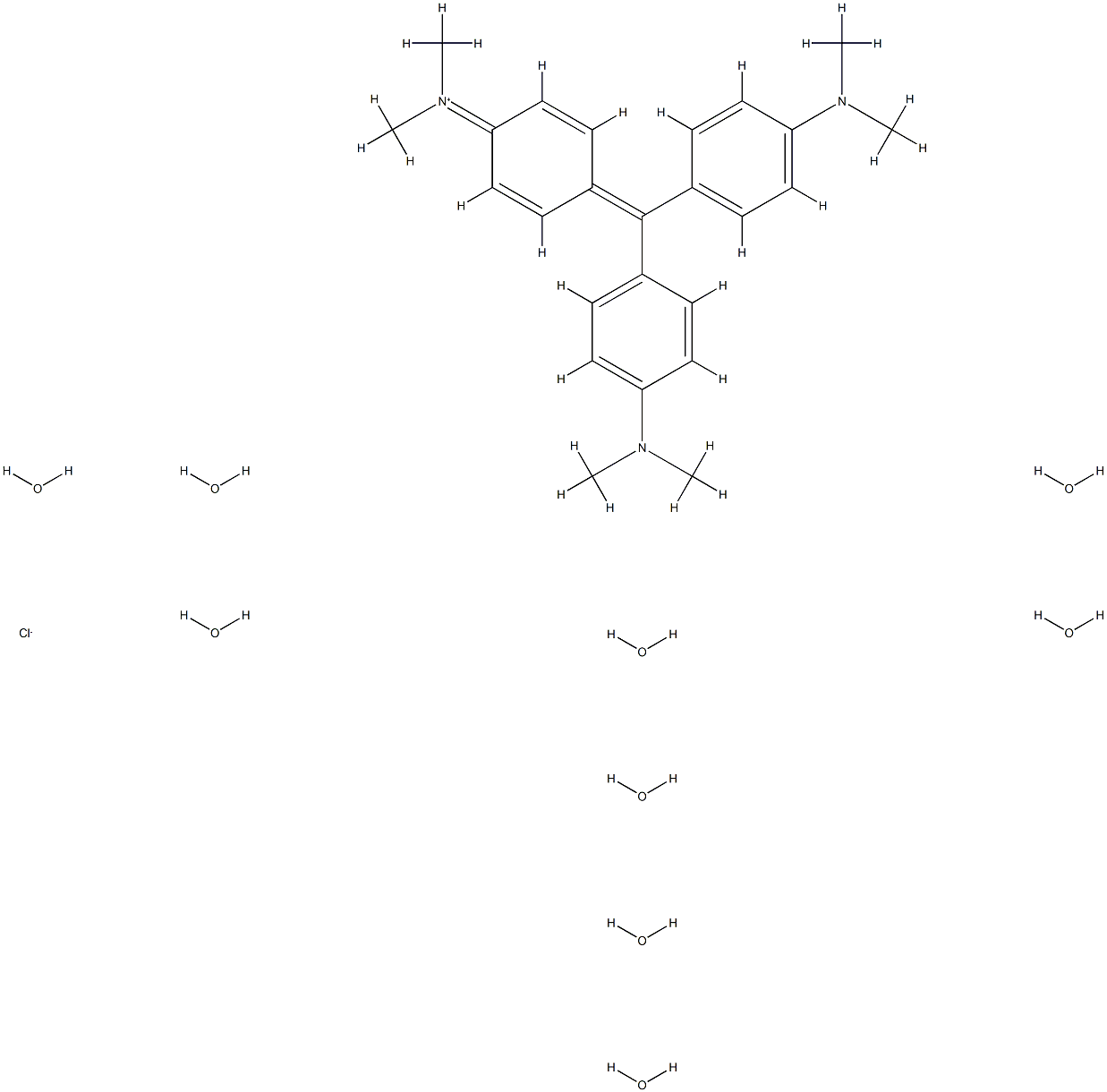
- Chemical Name:Crystal Violet Nonahydrate
- CAS:60662-33-1
- MF:C25H48ClN3O9
- Structure:
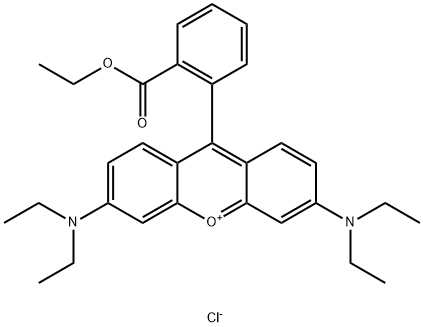
- Chemical Name:Basic Violet 11
- CAS:2390-63-8
- MF:C30H35ClN2O3
- Chemical Name:Basic Blue 62
- CAS:12221-36-2
- MF:
- Structure:
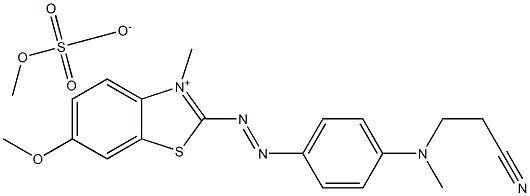
- Chemical Name:Basic Blue 162
- CAS:15085-91-3
- MF:C20H23N5O5S2
- Structure:
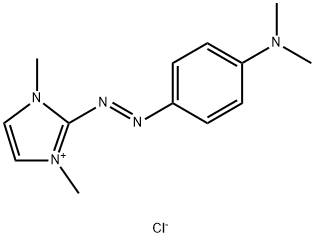
- Chemical Name:2-[[4-(dimethylamino)phenyl]azo]-1,3-dimethyl-1H-imidazolium chloride
- CAS:77061-58-6
- MF:C13H18ClN5
- Chemical Name:Basic Dyestuff
- CAS:
- MF:
- Chemical Name:PIGMENT VIOLET 3
- CAS:
- MF:
- Structure:
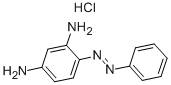
- Chemical Name:Basic Orange 2
- CAS:532-82-1
- MF:C12H13ClN4
- Structure:

- Chemical Name:BISMARCK BROWN B
- CAS:
- MF:C18H18N8
- Chemical Name:Basic Red 49
- CAS:12270-23-4
- MF:
- Chemical Name:Basic Yellow 29
- CAS:39279-59-9
- MF:C13H17ClN6O2
- Chemical Name:Basic Black
- CAS:
- MF:
- Structure:
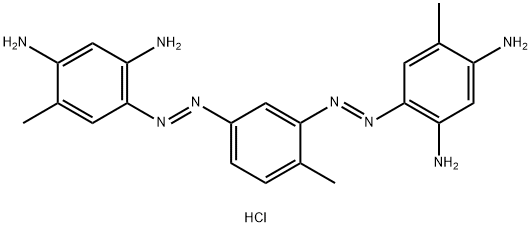
- Chemical Name:BISMARCK BROWN R
- CAS:5421-66-9
- MF:C21H25ClN8
- Structure:
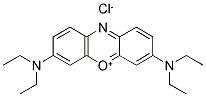
- Chemical Name:Phenazoxonium,3,7-bis(diethylamino)-,nitrate
- CAS:73570-52-2
- MF:C20H26N3O.NO3
- Chemical Name:Basic Brown 1
- CAS:8052-76-4
- MF:
- Structure:
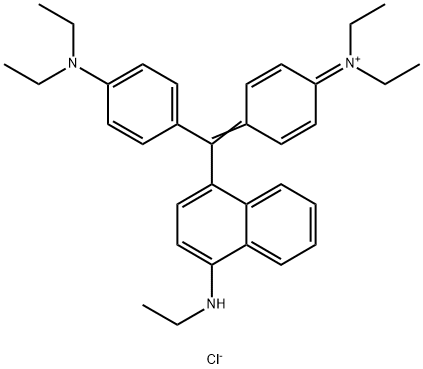
- Chemical Name:Basic Blue 7
- CAS:2390-60-5
- MF:C33H40ClN3
- Chemical Name:BASIC RED 15
- CAS:
- MF:C25H29Cl2N3
- Chemical Name:Basic Blue 53
- CAS:11075-20-0
- MF:C17H17I3N4SZn
- Structure:
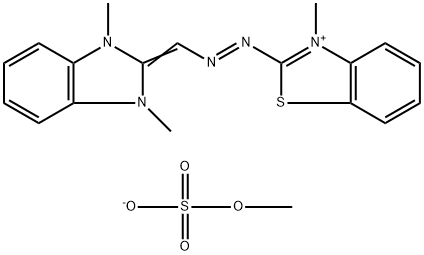
- Chemical Name:Basic Yellow 24
- CAS:52435-14-0
- MF:C19H21N5O4S2
- Chemical Name:C.I.Basic Blue 26
- CAS:
- MF:
- Structure:
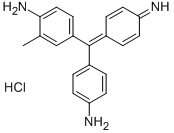
- Chemical Name:FUCHSIN BASIC
- CAS:632-99-5
- MF:C20H20ClN3
- Structure:
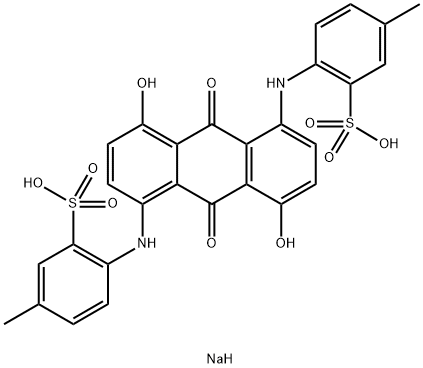
- Chemical Name:Toluidine bule
- CAS:3209-30-1
- MF:C28H23N2NaO10S2
- Chemical Name:Basic Red 54
- CAS:12270-28-9
- MF:
- Chemical Name:Basic Yellow 49
- CAS:55777-80-5
- MF:C14H17ClN4O
- Structure:
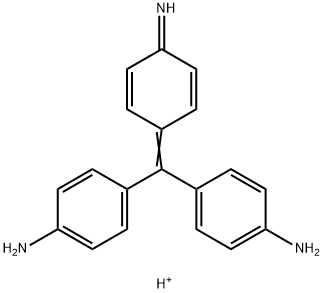
- Chemical Name:CI 42500
- CAS:25620-78-4
- MF:C19H18N3+
- Chemical Name:2 BASIC ORANGE 2
- CAS:
- MF:
- Structure:
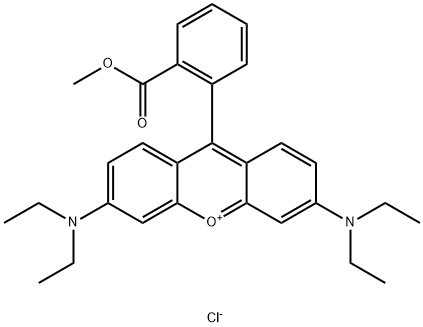
- Chemical Name:CRYSTAL VIOLET
- CAS:39393-39-0
- MF:C29H33ClN2O3
- Chemical Name:LIGNIN, ALKALI
- CAS:8068-05-1
- MF:C30H25ClN6
- Chemical Name:Basic Yellow 87
- CAS:116844-55-4
- MF:
- Chemical Name:Basic Scarlet G
- CAS:
- MF:
- Chemical Name:High alkali washing agent
- CAS:
- MF:
- Structure:
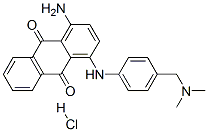
- Chemical Name:1-amino-4-[[4-[(dimethylamino)methyl]phenyl]amino]anthraquinone monohydrochloride
- CAS:67905-56-0
- MF:C23H22ClN3O2
- Chemical Name:28 BASIC YELLOW 28
- CAS:74504-47-5
- MF:
- Structure:
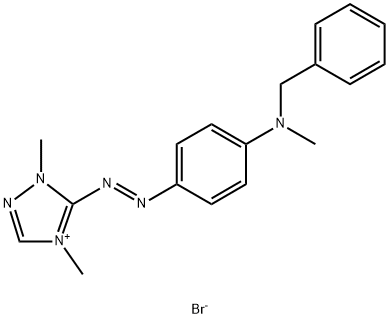
- Chemical Name:BASIC RED 46
- CAS:12221-69-1
- MF:C18H21BrN6
- Chemical Name:Basic Orange Piece
- CAS:
- MF:
- Structure:
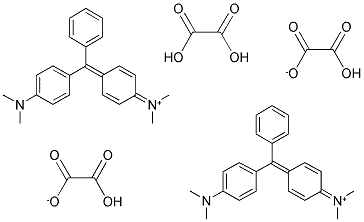
- Chemical Name:MALACHITE GREEN OXALATE
- CAS:13425-25-7
- MF:C23H25N2.C2HO4
- Structure:
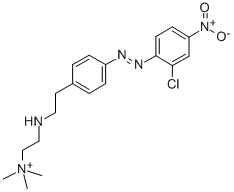
- Chemical Name:[2-[[4-[(2-chloro-4-nitrophenyl)azo]phenyl]ethylamino]ethyl]trimethylammonium
- CAS:14097-03-1
- MF:C19H25ClN5O2+
- Chemical Name:4 BASIC GREEN 4
- CAS:
- MF:
- Structure:
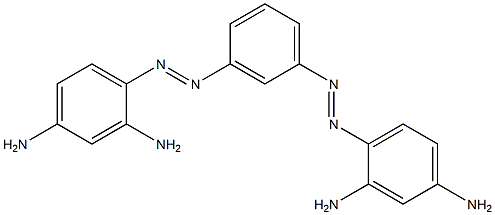
- Chemical Name:BismarkbrownG
- CAS:8005-77-4
- MF:C18H18N8
- Chemical Name:Basic Fruit Green
- CAS:
- MF:
- Structure:
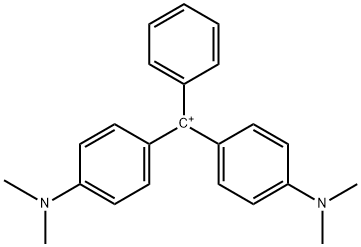
- Chemical Name:Magentagreencrystals
- CAS:14426-28-9
- MF:C23H25N2+
- Chemical Name:Basic Violet 11
- CAS:
- MF:
- Chemical Name:CATIONIC GREEN MELACHITE
- CAS:
- MF:
- Chemical Name:Basic Brown RC
- CAS:
- MF:
- Chemical Name:Basic Brown GN
- CAS:
- MF:
- Chemical Name:Basic Dyestuff
- CAS:
- MF:
- Chemical Name:Aizen
- CAS:
- MF:
- Chemical Name:Basic Blue 57
- CAS:12221-31-7
- MF:
- Structure:
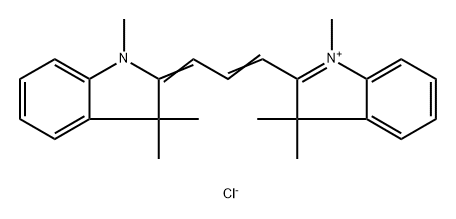
- Chemical Name:2-[3-(1,3-dihydro-1,3,3-trimethyl-2H-indol-2-ylidene)prop-1-enyl]-1,3,3-trimethyl-3H-indolium chloride
- CAS:6320-14-5
- MF:C25H29ClN2
- Structure:
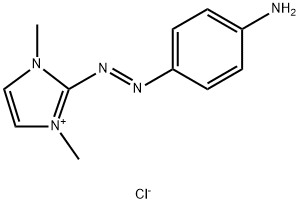
- Chemical Name:2-[(4-aminophenyl)azo]-1,3-dimethyl-1H-imidazolium chloride
- CAS:97404-02-9
- MF:C11H14ClN5
- Chemical Name:C.I.Basic Yellow 96
- CAS:167973-11-7
- MF:
- Structure:
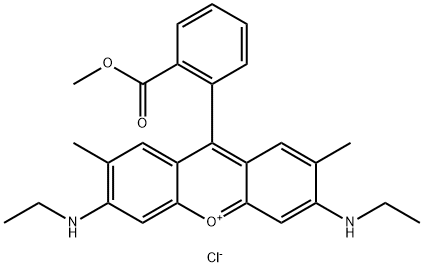
- Chemical Name:Basic Red 1:1
- CAS:3068-39-1
- MF:C27H29ClN2O3
- Structure:
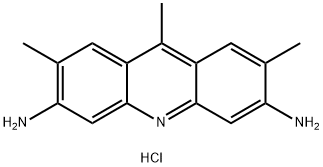
- Chemical Name:CI NO 46040
- CAS:4215-95-6
- MF:C16H18ClN3
- Structure:
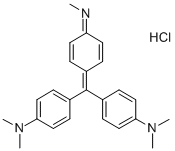
- Chemical Name:METHYL VIOLET
- CAS:603-47-4
- MF:C24H28ClN3
- Structure:

- Chemical Name:Basic Red 22
- CAS:12221-52-2
- MF:C15H21IN6
- Chemical Name:3(or5)-[[4-[benzylmethylamino]phenyl]azo]-1,2(or1,4)-dimethyl-1H-1,2,4-triazolium bromide
- CAS:89959-98-8
- MF:C18H21N6.Br
- Chemical Name:Alkaline Peptone Water
- CAS:
- MF:
- Structure:
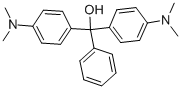
- Chemical Name:SOLVENT GREEN 1
- CAS:510-13-4
- MF:C23H26N2O
- Structure:
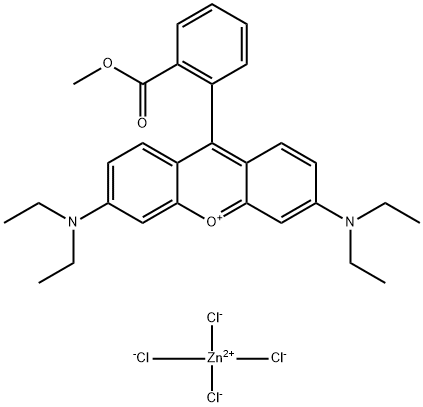
- Chemical Name:Basic Violet 11:1
- CAS:73398-89-7
- MF:C29H33Cl4N2O3Zn-
- Structure:
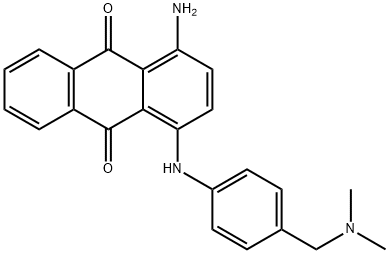
- Chemical Name:1-amino-4-[[4-[(dimethylamino)methyl]phenyl]amino]anthraquinone
- CAS:12217-43-5
- MF:C23H21N3O2
- Structure:
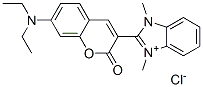
- Chemical Name:2-[7-(diethylamino)-2-oxo-2H-1-benzopyran-3-yl]-1,3-dimethyl-1H-benzimidazolium chloride
- CAS:29556-33-0
- MF:C22H24ClN3O2
- Chemical Name:18 BASIC RED 18
- CAS:
- MF:
- Chemical Name:Alkaline Agar
- CAS:
- MF: