-
물성
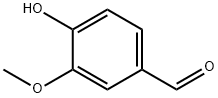
바닐린은 페놀 알데하이드, 분자 공식 C8H8O3를 가진 유기화합물입니다. 그것의 작용기는 알데하이드, 에테르 및 석탄산을 포함합니다. 바닐린은 바닐라 콩의 추출물의 1 차적인 성분입니다.
바닐린의 가장 큰 사용은 감미로운 음식에서 flavoring로, 보통 입니다. 아이스크림과 초콜렛 공업은 적은 양이 flavoring로 바닐린을 위한 시장을 함께 75%를, 과자와 구워진 상품에서 사용된 상태에서 함유합니다.
바닐린은 또한 많은 다른 풍미를 위해 아주 중요한 중요한 주로 풍미 공업에서, 특히 크림 같은 단면도 사용됩니다.
바닐린은 조제약과 다른 사람의 생산에 있는 화학물질 중간물로 벌금 화학물질 사용되었습니다.
-
생산/준비/합성
① 리그닌에서의 제조: 펄프 공업의 폐액 속에 함유된 리그닌 설폰산을 알칼리와 산화구리 또는 니트로벤젠으로 산화하여 리그닌바닐린을 얻는다.
② 구아야콜에서의 제조 : 구아야콜과 클로로포름 또는 클로랄을 알칼리 존재하에서 축합시키고(라이머-티만 반응), 이것을 산화시켜 합성한다.
③ 이 밖에 유제놀 또는 트리포린을 원료로 하는 방법도 있다.
또한 바닐린 분자속의 -OCH기(基) 대신에 -OCH기가 들어간 것이 합성되어 바닐린과 같은 방향을 가지고 또 그 강도도 강한 것이 나타나게 되었다.
이것을 에틸바닐린 또는 부르보날(bourbonal)이라고 한다.
바닐린에 비해 착색되지 않으므로 착색을 싫어하는 제품에 바닐린과 같은 용도, 달콤한 꽃향, 과일향 등 조합향료로 널리 사용된다.
-
용도
그것은 화장품 것과 같이 그리고 고무, 플라스틱 및 다른 제품의 향수를 바르기에서 제과, 아이스크림, 음료, 케이크, 초콜렛, 빵, 건빵, 담배, 포도주 및 사료 우물에서 널리 이용됩니다. 그것은 또한 약에서 사용되고, 그리고 다른 기업 그리고 화학 시약으로 사용해 전기도금을 합니다.
-
순도시험
(1) 융점 : 이 품목의 융점은 81~83℃이어야 한다.
(2) 용상 : 이 품목 1g에 물 20mL를 가하여 80℃에서 가열하여 녹일 때, 그 액은 징명하여야 한다.
(3) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(4) 납 : 이 품목 5.0g을 취하여 원자흡광광도법 또는 유도결합플라즈마발광광도법에 따라 시험할 때, 그 양은 2.0ppm 이하이어야 한다.
-
확인시험
(1) 이 품목의 포화수용액 10mL에 염화제이철시액 3방울을 가하면 청자색을 나타낸다. 다음에 이를 약 80℃에서 5분간 가열하면 갈색이 되고 백~회백색의 침전이 생긴다.
(2) 이 품목 1g에 아황산수소나트륨시액 5mL를 가하고 온탕 중에서 가온하면서 흔들어 섞어 녹이고 이에 묽은황산 10mL를 가하여 60~70℃에서 약 5분간 가온한 다음 방치하면 결정이 석출한다.
-
정량법
이 품목 1g을 정밀히 달아 향료시험법 중 알데히드류 및 케톤류함량측정법 (3) 히드록실아민법 제2법에 따라 정량한다. 단, 방치시간은 15분간으로 한다.
0.5N 염산 1mL = 76.07mg C8H8O3
-
강열잔류물
이 품목의 강열잔류물은 0.05% 이하이어야 한다.
-
개요
Vanillin is found in many plants, such as the tuber of Rhizoma Gastrodiae (Tian
Ma), the whole herb of Equisetum (Mu Zei), Ulva pertusa (Kong Shi Chun), and
sugar beets, vanilla beans, Peru balsam, and so on .
-
화학적 성질
Vanillin is found in many essential oils and
foods but is often not essential for their odor or aroma. However, it does determine
the odor of essential oils and extracts from Vanilla planifolia and Vanilla
tahitensis pods, in which it is formed during ripening by enzymatic cleavage of
glycosides.
Vanillin is a colorless, crystalline solid (mp 82–83°C) with a typical
vanilla odor. Because it possesses aldehyde and hydroxy substituents, it undergoes
many reactions. Additional reactions are possible due to the reactivity of the aromatic
nucleus. Vanillyl alcohol and 2-methoxy-4-methylphenol are obtained by
catalytic hydrogenation; vanillic acid derivatives are formed after oxidation and
protection of the phenolic hydroxy group. Since vanillin is a phenol aldehyde, it is
stable to autoxidation and does not undergo the Cannizzaro reaction. Numerous
derivatives can be prepared by etherification or esterification of the hydroxy group
and by aldol condensation at the aldehyde group. Several of these derivatives are
intermediates, for example, in the synthesis of pharmaceuticals.
-
물리적 성질
Appearance: white or light yellow needle crystal or crystal powder, with a strong
aroma. The relative density is about 1.060. Solubility: It is not only soluble in ethanol, chloroform, ether, carbon disulfide, glacial acetic acid, and pyridine but also in
oil, propylene glycol, and hydrogen peroxide in alkaline solution. It can slowly
oxidize in the air, can be unstable under illumination, and should be stored in a dark
condition. Melting point: the melting point is 81°C.
-
출처
Vanillin occurs widely in nature; it has been reported in the essential oil of Java citronella (Cymbopogon nardus
Rendl.), in benzoin, Peru balsam, clove bud oil and chiefly vanilla pods (Vanilla planifolia, V. tahitensis, V. pompona); more that
40 vanilla varieties are cultivated; vanillin is also present in the plants as glucose and vanillin. Reported found in guava, feyoa fruit,
many berries, asparagus, chive, cinnamon, ginger, Scotch spearmint oil, nutmeg, crisp and rye bread, butter, milk, lean and fatty fish,
cured pork, beer, cognac, whiskies, sherry, grape wines, rum, cocoa, coffee, tea, roast barley, popcorn, oatmeal, cloudberry, passion
fruit, beans, tamarind, dill herb and seed, sake, corn oil, malt, wort, elderberry, loquat, Bourbon and Tahiti vanilla and chicory root.
-
역사
Vanillin is known as one of the first synthetic spices. In the perfume industry, it is
known as vanillic aldehyde. As early as 1858, French chemist Gby (NicolasTheodore Gobley) obtained pure vanillin for the first time by the method of
rectification. Due to less production yield of natural vanillin, it spurred the search
for a chemical synthesis method of vanillin production. In 1874, German scientist M.?Haarman and co-workers deduced the chemical structure of vanillin and discovered a new way to produce vanillin with abietene as the raw material . In
1965, Chinese scientists found that vanillin has antiepileptic effect and accomplished a study on the pharmacology and toxicology of vanillin from edible to
officinal. They also found that vanillin has certain antibacterial activity, making it
a suitable drug formulation for the treatment of skin disease. Vanillin can be used
as intermediate for synthesis of a variety of drugs, such as berberine and antihypertensive drug L-methyldopa, methoxy-pyrimidine, and heart disease drug
papaverine .
-
용도
Labelled Vanillin. Occurs naturally in a wide variety of foods and plants such as orchids; major commercial source of natural vanillin is from vanilla bean extract. Synthetically produced in-bulk fro
m lignin-based byproduct of paper processes or from guaicol.
-
정의
ChEBI: A member of the class of benzaldehydes carrying methoxy and hydroxy substituents at positions 3 and 4 respectively.
-
Indications
It can be used to treat various types of epilepsy and attention deficit hyperactivity
disorder and vertigo.
-
생산 방법
Vanillin occurs naturally in many essential oils and particularly in
the pods of Vanilla planifolia and Vanilla tahitensis. Industrially,
vanillin is prepared from lignin, which is obtained from the sulfite
wastes produced during paper manufacture. Lignin is treated with
alkali at elevated temperature and pressure, in the presence of a
catalyst, to form a complex mixture of products from which vanillin
is isolated. Vanillin is then purified by successive recrystallizations.
Vanillin may also be prepared synthetically by condensation, in
weak alkali, of a slight excess of guaiacol with glyoxylic acid at
room temperature. The resultant alkaline solution, containing 4-
hydroxy-3-methoxymandelic acid is oxidized in air, in the presence
of a catalyst, and vanillin is obtained by acidification and
simultaneous decarboxylation. Vanillin is then purified by successive
recrystallizations.
-
일반 설명
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to pharmacopeia primary standards
-
공기와 물의 반응
Slowly oxidizes on exposure to air. . Slightly water soluble.
-
반응 프로필
Vanillin can react violently with Br2, HClO4, potassium-tert-butoxide, (tert-chloro-benzene + NaOH), (formic acid + Tl(NO3)3). . Vanillin is an aldehyde. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction).
-
화재위험
Flash point data for Vanillin are not available, however Vanillin is probably combustible.
-
Pharmaceutical Applications
Vanillin is widely used as a flavor in pharmaceuticals, foods,
beverages, and confectionery products, to which it imparts a
characteristic taste and odor of natural vanilla. It is also used in
perfumes, as an analytical reagent and as an intermediate in the
synthesis of a number of pharmaceuticals, particularly methyldopa.
Additionally, it has been investigated as a potential therapeutic
agent in sickle cell anemia and is claimed to have some antifungal
properties.
In food applications, vanillin has been investigated as a
preservative.
As a pharmaceutical excipient, vanillin is used in tablets,
solutions (0.01–0.02% w/v), syrups, and powders to mask the
unpleasant taste and odor characteristics of certain formulations,
such as caffeine tablets and polythiazide tablets. It is similarly used
in film coatings to mask the taste and odor of vitamin tablets.
Vanillin has also been investigated as a photostabilizer in
furosemide 1% w/v injection, haloperidol 0.5% w/v injection, and
thiothixene 0.2% w/v injection.
-
Clinical Use
Vanillin tablet has been used in the treatment of epilepsy and has a better therapeutic
effect. Some patients have a minor dizziness response occasionally in the clinic.
-
Safety Profile
Moderately toxic by
ingestion, intraperitoneal, subcutaneous, and
intravenous routes. Experimental
reproductive effects. Human mutation data
reported. Can react violently with Br2,
HClO4, potassium-tert-butoxide, tert-
chlorobenzene + NaOH, formic acid +
thallium nitrate. When heated to
decomposition it emits acrid smoke and
irritating fumes. See also ALDEHYDES.
-
Safety
There have been few reports of adverse reactions to vanillin,
although it has been speculated that cross-sensitization with other
structurally similar molecules, such as benzoic acid, may occur.
Adverse reactions that have been reported include contact
dermatitis and bronchospasm caused by hypersensitivity.
The WHO has allocated an estimated acceptable daily intake for
vanillin of up to 10 mg/kg body-weight.
LD50 (guinea pig, IP): 1.19 g/kg
LD50 (guinea pig, oral): 1.4 g/kg
LD50 (mouse, IP): 0.48 g/kg
LD50 (rat, IP): 1.16 g/kg
LD50 (rat, oral): 1.58 g/kg
LD50 (rat, SC): 1.5 g/kg
-
신진 대사
Early observers noted conversion of vanillin to vanillic acid which was excreted mainly as the free acid, a conjugated ethereal sulphate or glucurovanillic acid (Preusse, 1880). In man, vanillin is broken down by the liver to vanillic acid which is excreted in the urine. Human liver homogenates readily convert vanillin to vanillic acid in vitro (Dirscherl & Brisse, 1966). Endo�genous vanillic acid production and excretion in man from body catecholamines amounts to <0.5 mg/day, compared with the normal contribution from dietary sources of about 9 mg/day (Dir�scherl & Wirtzfeldt, 1964).
-
저장
Vanillin oxidizes slowly in moist air and is affected by light.
Solutions of vanillin in ethanol decompose rapidly in light to give
a yellow-colored, slightly bitter tasting solution of 6,6’-dihydroxy-
5,5’-dimethoxy-1,1’-biphenyl-3,3’-dicarbaldehyde. Alkaline solutions
also decompose rapidly to give a brown-colored solution.
However, solutions stable for several months may be produced by
adding sodium metabisulfite 0.2% w/v as an antioxidant.
The bulk material should be stored in a well-closed container,
protected from light, in a cool, dry place.
-
Purification Methods
Crystallise vanillin from water or aqueous EtOH, or by distillation in vacuo.[Beilstein 8 IV 1763.]
-
비 호환성
Incompatible with acetone, forming a brightly colored compound. A compound practically insoluble in ethanol is formed
with glycerin.
-
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database
(oral solutions, suspensions, syrups, and tablets). Included in nonparenteral medicines licensed in the UK. Included in the
Canadian List of Acceptable Non-medicinal Ingredients.