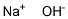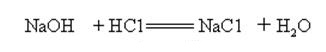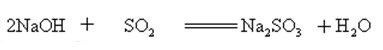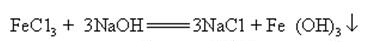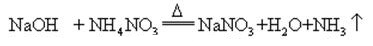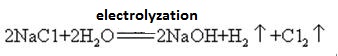-
물성
• 알칼리성으로 백색 반투명의 조해성 고체이며 후레이크 편상으로 되어있슴
• 수용액은 강염기성이며 물에 용해되며 발열하며 비누물과 같이 미끈미끈한 감촉을 나타내는데 대단히 부식성이 강함
-
용도
바이오디젤
바이오디젤을 생산할 때 수산화 나트륨은 교환반응의 촉매로 사용된다. 물과 지방은 비누화반응을 일으키기 때문에 무수수산화나트륨만 이용할 수 있다. 더 싸고 적은 양이 필요하기 때문에 수산화 칼륨에 비해 자주 쓰인다.
마약 제조
수산화 나트륨은 메스암페타민이나 다른 마약을 제조하는 데 필수적인 물질이다. 언론에 널리 알려진 것과 다르게 이것은 제조 원료로서 들어가는 것은 아니며 단지 화학 반응상 pH를 조절하기 위한 강염기로 사용되는 것이다.
-
용도
이것은 인견, 종이, 펄프, 비누, 합성섬유등의 제조를 위한 원료로 이용되고 있고, 산성의 처리제, 산성용액의 중화제로 사용되며 염료의 중간물, 향료, 농약, 의약품 제조, 유지의 정제에 사용된다.
식품 첨가물로서는 식품제조시 알칼리제, 중화제로 사용된다.
-
용도
인견, 인조섬유, 셀로판, 합성섬유의 제조, 염료중간물, 향료, 의약품, 면사 및 면포의 정련, 유지의 정제, 비누등의 제조, 종이와 펄프제조, 석유 타르유정제, 알루미나, 각종 소다 염류의 제조, 물의 연화제, 알칼리 축전지의 전기분해액, 일반세정용, 중화, 분석용시약, 염료중간물, 농약, 악취제거 등
-
순도시험
(1) 용상 : 이 품목 50g을 새로 끓여서 식힌 물에 녹여 250mL로 하여 시험용액으로 한다. 이 액 5mL에 물 20mL를 가하여 섞을 때, 그 액은 무색으로서 탁도는 거의 징명 이하이어야 한다.
(2) 탄산나트륨 : 정량법에서 얻은 탄산나트륨(Na2CO3)의 함량은 2% 이하이어야 한다.
(3) 비소 : 이 품목을 비소시험법에 따라 시험할 때, 그 양은 4.0ppm 이하이어야 한다.
(4) 납 : 「메타인산나트륨」의 순도시험 (2)에 따라 시험한다(0.5ppm 이하).
(5) 수은 : 위 (1)의 시험용액 10mL를 취하여 과망간산칼륨용액(3→50) 1mL 및 물 약 30mL를 가해 흔들어 녹인다. 이 액에 정제염산을 서서히 가하여 중화하고 다시 황산(1→2) 5mL를 가하고 식힌 다음 이를 시험용액으로 한다. 다음 시험용액중의 과망간산칼륨의 자색이 없어지고 또 이산화망간의 침전이 녹을 때까지 염산히드록실아민용액(1→5)을 가한 다음 물을 가하여 100mL로 하고 원자흡광분석장치의 검수병에 넣는다. 다시 염화제일주석시액 10mL를 가하고 즉시 원자흡광분석장치를 연결하고 다이야푸람펌프를 작동시켜서 공기를 순환시켜 기록계의 지시가 급속히 상승하여 일정치를 나타낼 때의 흡광도는 수은표준용액 2mL, 과망간산칼륨용액(3→50) 1mL, 물 약 30mL 및 시험용액 처리에 사용한 양의 정제염산을 가하여 검체와 같이 조작하여 얻은 흡광도보다 커서는 아니 된다(0.1ppm 이하).
-
확인시험
(1) 이 품목의 수용액(1→50)은 강알칼리성이다.
(2) 이 품목의 수용액(1→25)은 확인시험법 중 나트륨염의 반응을 나타낸다.
-
정량법
이 품목 약 50g을 정밀히 달아 새로 끓여서 식힌 물에 녹여 1,000mL로 하여 시험용액으로 한다. 그 중 25mL에 새로 끓여서 식힌 물 10mL를 가하고 브로모페놀블루시액 1mL를 지시약으로 하여 1N 염산으로 적정하여 중화점에 달하면 다시 1N 염산 약 1mL를 더 가하여 약 5분간 끓인 다음 식히고 0.1N 수산화나트륨용액으로 과잉의 산을 적정하여 1N 염산의 소비량 AmL를 구한다. 따로 시험용액 25mL를 공전플라스크에 취하여 새로 끓여서 식힌 물 25mL를 가하고 염화바륨시액 10mL를 가하여 마개를 막고 조용히 흔들어 섞어 페놀프탈레인시액 1mL를 지시약으로 하여 1N 염산을 적정하고 그 소비량을 BmL로 한다.
-
정의
이 품목에는 결정물 및 무수물이 있고, 각각을 수산화나트륨(결정) 및 수산화나트륨(무수)이라 칭한다. 결정물은 수산화나트륨(무수)과 수산화나트륨(1수염)의 혼합물이다.
-
개요
아세트산은 체내에서 당, 아미노산, 지방 등의 대사의 결과 생성되기도 한다.
알코올성 음료를 마시면 체내에 에탄올이 들어오게 되는데, 이는 알코올 수소이탈효소에 의하여 아세트알데하이드가 된다. 아세트알데하이드는 다시 알데하이드 수소이탈효소 등에 의해 아세트산으로 바뀌게 된다. 이렇게 해서 생성된 아세트산은 아세틸-CoA 등으로 바뀌어 TCA 회로에 투입되어 ATP 생산에 사용되거나 다른 대사과정에 사용된다.
-
화학적 성질
Sodium hydroxide, NaOH,also referred to as caustic soda or sodium hydrate(and formerly known as lye), is a white,massive, deliquescent crystalline solid that is soluble in water,alcohol, and glycerol. It melts at 318°C (606 OF) and is the most widely used and available alkaline chemical. Most sodium hydroxide is produced as a coproduct of chlorine through the use of electrolytic cells;the cells are of the diaphragm, mercury, or membrane type. Some sodium hydroxide is marked as produced in the cells;most is evaporated and sold as 50% and 73% solutions or as anhydrous beads. Most caustic end uses require solutions of relatively low concentrations. Caustic soda is used as an analytical reagent and chemical intermediate, in scouring and cleaning baths,in rubber reclaiming and petroleum refining, in quenching baths for heat treating of steel,in cutting and soluble oils,in soaps and detergents, and in a wide variety of other applications.
-
물리적 성질
White orthorhombic crystals, produced in the form of pellets, lumps, sticks, beads, chips, flakes or solutions; hygroscopic; very corrosive; rapidly absorbs CO2 and water from the air; density 2.13 g/cm3; melts at 323°C; vaporizes at 1388°C; vapor pressure 1 torr at 739°C and 5 torr at 843°C; very soluble in water (110 g/100mL at room temperature), generating heat on dissolution; aqueous solutions highly alkaline, pH of 0.5% solution about 13 and 0.05% solution about 12; soluble in methanol, ethanol and glycerol (23.8 g/100 mL methanol and 13.9 g/100 mL ethanol at ambient temperatures.).
-
용도
Sodium hydroxide is one of the most important industrial chemicals. In volume, it is in the top ten chemicals produced in the United States. It is used in manufacturing a large number of compounds including several sodium salts, in treating cellulose for producing rayon and cellophane, and in manufacturing soaps, detergents, pulp, and paper. Sodium hydroxide is a common neutralizing agent for acids in acid-base titrations and petroleum refining. Another major application is extracting metals from their ores where alkali fusion, such as fusion with caustic soda, often is applied to open the ores. Additionally, sodium hydroxide is used to precipitate metals as hydroxides. Other uses are in reclaiming rubber, dissolving casein in plastics production, refining vegetable oils, processing textiles, as an eluant in ion chromatography, etching and electroplating, and as a laboratory reagent. Sodium hydroxide also is used as a strong base in many organic synthesis and base-catalyzed reactions.
-
정의
The most important commercial
caustic.
-
생산 방법
Sodium hydroxide is manufactured by electrolysis of brine using
inert electrodes. Chlorine is evolved as a gas at the anode and
hydrogen is evolved as a gas at the cathode. The removal of chloride
and hydrogen ions leaves sodium and hydroxide ions in solution.
The solution is dried to produce the solid sodium hydroxide.
A second method uses the Kellner–Solvay cell. Saturated sodium
chloride solution is electrolyzed between a carbon anode and a
flowing mercury cathode. In this case the sodium is produced at the
cathode rather than the hydrogen because of the readiness of
sodium to dissolve in the mercury. The sodium–mercury amalgam is
then exposed to water and a sodium hydroxide solution is
produced.
-
화학 반응
Sodium hydroxide is strongly alkaline and can react with acids to form salts and water.
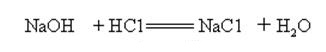
Sodium hydroxide reacts with acidic oxides to form salt and water, so sodium hydroxide can be used to absorb acid gases in the laboratory or industrially.
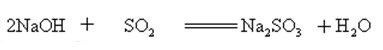
Sodium hydroxide can react with aqueous solutions of many metal salts to form sodium salts and metal hydroxides
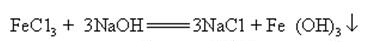
When sodium hydroxide and ammonia salt are heated together, it can release ammonia
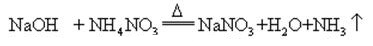
Sodium hydroxide is highly corrosive, so that the glass bottles storing sodium hydroxide solutions must be rubber stoppers, and glass stoppers should not be used to prevent a chemical reaction from opening. Sodium hydroxide is an important industrial raw material, and can be produced by electrolysis of saline solution industrially
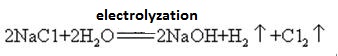
-
안전성
고체 수산화 나트륨이나 고농도의 수산화 나트륨 수용액은 화학적 화상을 유발할 수 있으며 영구적인 상처나 실명을 유발할 수 있다.
알루미늄과 수산화 나트륨은 많은 수소 기체를 발생시킨다.
2Al(s) + 6NaOH(aq) → 3H2(g) + 2Na3AlO3(aq).
밀폐된 공간에서 수소 기체를 대량으로 발생시킬 경우 위험하다.
-
일반 설명
A white solid. Corrosive to metals and tissue. Used in chemical manufacturing, petroleum refining, cleaning compounds, drain cleaners.
-
공기와 물의 반응
Soluble in water. Dissolution can liberate enough heat to cause steaming and spattering and ignite adjacent combustible material [Haz. Chem. Data 1966].
-
위험도
Corrosive to tissue in presence of mois-
ture, strong irritant to tissue (eyes, skin, mucous
membranes, and upper respiratory tract), poison by
ingestion.
-
건강위험
Sodium hydroxide is a highly corrosive substancethat causes damage to human tissues.Its action on the skin is somewhat differentfrom acid burns. There is no immediate pain,but it penetrates the skin. It does not coagulateprotein to prevent its further penetration,and thus the caustic burn can become severeand slow healing. Spilling of its concentratedsolutions into the eyes can result in severeirritation or permanent injury.
It is toxic by ingestion as well as inhalationof its dust. Although the oral toxicity ofa 5–10% solution of caustic soda was foundto be low in test animals, high dosages atgreater concentrations can cause vomiting,prostration, and collapse. The oral lethal dosein rabbits is 500 mg/kg (NIOSH 1986).
Sodium hydroxide dusts or aerosols areirritating to the eyes, nose, and throat. Prolongedexposure to high concentrations in airmay produce ulceration of the nasal passage.
-
화재위험
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
-
인화성 및 폭발성
Sodium hydroxide and potassium hydroxide are not flammable as solids or aqueous
solutions.
-
Pharmaceutical Applications
Sodium hydroxide is widely used in pharmaceutical formulations to
adjust the pH of solutions. It can also be used to react with weak
acids to form salts.
-
공업 용도
Caustic soda (NaOH) is regarded as the strongest alkaline pH regulator. Caustic soda
is a very active substance and is highly corrosive. The bulk of caustic soda is manufactured
by electrolysis of saturated brines (NaCl). Caustic soda has a very strong pHregulating
capability (i.e. from pH 7 to pH 14) at a relatively low dosage compared to
other alkaline substances. Commercially, caustic soda is available in anhydrous form,
but in most mining applications the caustic soda is supplied as a 50% solution.
In the mineral processing industry, sodium hydroxide is mostly used for alkalinity control
during the processing of non-metallic minerals. In base metal flotation, the use of
sodium hydroxide is rare.
-
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. Mutation data reported. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of NanO.
-
Safety
Sodium hydroxide is widely used in the pharmaceutical and food
industries and is generally regarded as a nontoxic material at low
concentrations. At high concentrations it is a corrosive irritant to
the skin, eyes, and mucous membranes.
LD50 (mouse, IP): 0.04 g/kg
LD50 (rabbit, oral): 0.5 g/kg
-
잠재적 노출
NaOH is utilized to neutralize acids and make sodium salts in petroleum refining, viscose rayon; cellophane, plastic production; and in the reclamation of solutions of their salts. It is used in the manufacture of mercerized cotton, paper, explosives, and dyestuffs in metal cleaning; electrolytic extraction of zinc; tin plating; oxide coating; laundering, bleaching, dishwashing; and it is used in the chemical industries.
-
저장
Sodium hydroxide should be stored in an airtight nonmetallic
container in a cool, dry place. When exposed to air, sodium
hydroxide rapidly absorbs moisture and liquefies, but subsequently
becomes solid again owing to absorption of carbon dioxide and
formation of sodium carbonate.
-
운송 방법
UN1823 NaOH, solid, Hazard class: 8; Labels: 8-Corrosive material. UN1824 NaOH, solution, Hazard class: 8; Labels: 8-Corrosive material
-
비 호환성
Sodium hydroxide is a strong base and is incompatible with any
compound that readily undergoes hydrolysis or oxidation. It will
react with acids, esters, and ethers, especially in aqueous solution.
-
폐기물 처리
Discharge into tank containing water, neutralize, then flush to sewer with water.
-
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (dental
preparations; injections; inhalations; nasal, ophthalmic, oral, otic,
rectal, topical, and vaginal preparations). Included in nonparenteral
and parenteral medicines licensed in the UK. Included in the
Canadian List of Acceptable Non-medicinal Ingredients.