ChemicalBook > CAS DataBase List > Ampicillin
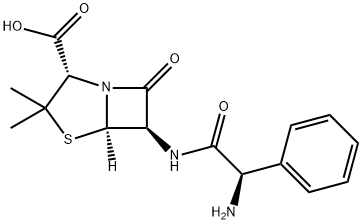
Ampicillin
Ampicillin
- CAS No.69-53-4
- Chemical Name:Ampicillin
- CBNumber:CB2247346
- Molecular Formula:C16H19N3O4S
- Formula Weight:349.4
- MOL File:69-53-4.mol
Ampicillin Property
- Melting point 198-200 °C (dec.)(lit.)
- alpha D23 +287.9° (c = 1 in water)
- Boiling point 201.8°C (rough estimate)
- Density 1.2794 (rough estimate)
- refractive index 1.6320 (estimate)
- storage temp. 2-8°C
- solubility NH4OH 1 M: 50 mg/mL, clear, colorless
- form solid
- color White to Pale Yellow
- pka 2.5 (COOH)(at 25℃)
- optical activity +287.923 (c 1.0, H2O)
- biological source microbial
- Water Solubility 13.9g/L(25 ºC)
- Merck 13,591
- JECFA Number 85
- BRN 1090925
- Stability Stable, but may be moisture sensitive. Incopmpatible with strong oxidizing agents.
- InChIKey AVKUERGKIZMTKX-NJBDSQKTSA-N
- CAS DataBase Reference 69-53-4(CAS DataBase Reference)
- FDA 21 CFR 556.40
- FDA UNII 7C782967RD
- NCI Drug Dictionary Wymox
- ATC code J01CA01,J01CA51,S01AA19
- IARC 3 (Vol. 50) 1990
- EPA Substance Registry System Ampicillin (69-53-4)
- UNSPSC Code 51280000
- NACRES NA.76
Safety
- Hazard Codes :Xn,T
- Risk Statements :36/37/38-42/43-61
- Safety Statements :22-26-36/37-45-53
- WGK Germany :2
- RTECS :XH8425000
- F :10-23
- Autoignition Temperature :301°C
- HS Code :29212900
- Hazardous Substances Data :69-53-4(Hazardous Substances Data)
- Toxicity :LD50 oral in mouse: > 5gm/kg
-
Symbol(GHS)

- Signal wordDanger
- Hazard statements H317-H334
- Precautionary statements P261-P272-P280-P284-P302+P352-P333+P313
Ampicillin Price
More Price(8)
- Brand: Sigma-Aldrich(India)
- Product number: A9393
- Product name : Ampicillin
- Purity: anhydrous, 96.0-102.0% (anhydrous basis)
- Packaging: 5G
- Price: ₹9807.45
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: PHR2838
- Product name : Ampicillin
- Purity: pharmaceutical secondary standard, certified reference material
- Packaging: 500MG
- Price: ₹16724.63
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: A9393
- Product name : Ampicillin
- Purity: anhydrous, 96.0-102.0% (anhydrous basis)
- Packaging: 25G
- Price: ₹35008.05
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: A5354
- Product name : Ampicillin
- Purity: Ready Made Solution, 100?mg/mL, 0.2 μm filtered
- Packaging: 10ML
- Price: ₹10879.4
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 59349
- Product name : Ampicillin
- Purity: analytical standard
- Packaging: 100MG
- Price: ₹5228.48
- Updated: 2022/06/14
- Buy: Buy
Ampicillin Chemical Properties,Usage,Production
-
Brand Name(s)
Human forms include Omnipen, Principen, Totacillin, Polycillin. Injectable forms include Omnipen-N, Polycillin-N, TotacillinN.
Veterinary forms include Amp-Equine, and Ampicillin trihydrate (Polyflex). -
Description
Ampicillin is an antibacterial antibiotic from the α-aminobenzyl penicillin group, which differs from penicillin by the presence of an amino group that facilitates penetration through the outer membrane of some gram-negative bacteria.
Ampicillin is Semi-synthetic derivative of penicillin that functions as an orally active broad-spectrum antibiotic. Ampicillin acts by interfering directly with the biosynthesis of peptidoglycan, which constitutes the major component of the bacterial cell wall, leading to structural instability and death of bacteria.
Ampicillin is a β-lactam antibiotic within the penicillin family. As a member of this family, Ampicillin is susceptible to β-lactamase, which hydrolyzes the β-lactam ring. This broad spectrum antibiotic is effective against gram-positive, gram-negative bacteria and anaerobic bacteria. Ampicillin is widely used in cell culture as a selective agent. As an antibiotic, Ampicillin binds to penicillin binding proteins (PBPs) on a susceptible organism and Inhibits bacterial cell-wall synthesis by inactivating transpeptidases on the inner surface of the bacterial cell membrane. After binding to the PBPs, Ampicilin acts as a structural analogue of acyl-D-alanyl-D-alanine, acylates the transpeptidase enzyme and thereby prevents the cross-linking of the peptidoglycan of the cell wall necessary for the growth of the bacterium.
Ampicillin sodium can be used as a selective agent in several types of isolation media. Ampicillin sodium is routinely used to select for cells containing the pcDNA3.1 and pEAK10 resistance plasmids in cell line A904L at an effective concentration of 50 μg/ml. - Chemical Properties Crystalline Solid
- Chemical Properties Ampicillin in anhydrous form occurs as crystals.
- Originator Binotal,Bayer,W. Germany,1962
- Uses β-lactam antibiotics
- Uses Ampicillin caused contact dermatitis in a nurse also sensitized to amoxicillin (with tolerance to oral phenoxymethylpenicillin), and in a pharmaceutical factory worker.
- Uses Labelled Ampicillin. Orally active, semi-synthetic antibiotic; structurally related to penicillin. Antibacterial.
- Definition ChEBI: A penicillin in which the substituent at position 6 of the penam ring is a 2-amino-2-phenylacetamido group.
-
Manufacturing Process
α-Carbobenzyloxyaminophenylacetic acid (0.1 mol), which is obtained by the
reaction of equivalent quantities of α-aminophenylacetic acid and benzyl
chlorocarbonate in aqueous sodium hydroxide, dissolved in dry acetone is
stirred and cooled to approximately -5°C. To this there is added dropwise with
continued cooling and stirring a solution of ethyl chlorocarbonate (0.1 mol).
After approximately 10 minutes, the acylating mixture is cooled to about -5°C
and then is slowly added to a stirred ice-cold mixture of 6-aminopenicillanic
acid (0.1 mol), 3% sodium bicarbonate solution (0.1 mol) and acetone. This
reaction mixture is allowed to attain room temperature, stirred for an
additional thirty minutes at this temperature and then is extracted with ether.
The extracted aqueous solution is covered with butanol and the pH adjusted to 2 by the addition of HCl. The acidified aqueous phase is extracted with butanol, the pH of the aqueous phase being adjusted to pH 2 each time. The combined butanol solutions which contain the free acid, α- carbobenzyloxyaminobenzylpenicillin, are washed with water, and are then shaken with water to which sufficient 3% sodium bicarbonate has been added to bring the aqueous phase to pH 7. The process of washing and shaking is repeated with fresh water and bicarbonate solution. The combined aqueous solutions are washed with ether and then are evaporated under reduced pressure and low temperature. The product, the sodium salt of α- carbobenzyloxyaminobenzylpenicillin, is obtained as a yellow solid in a yield of 65%.
A suspension of palladium on barium carbonate (3.7 grams of 30%) in water (20 ml) is shaken in an atmosphere of hydrogen at room temperature. The catalyst is then filtered and washed well with water, care being taken that it does not become dry. A solution of the sodium salt of α- carbobenzyloxyaminobenzylpenicillin (4 grams) in water (20 ml) is added to the pretreated catalyst and the suspension is shaken in an atmosphere of hydrogen at room temperature and pressure for one hour. The catalyst is then filtered off, washed well with water, and the combined filtrate and washings adjusted to pH 7 with hydrochloric acid. The resulting solution is evaporated in vacuum at a temperature below 20°C to give α-aminobenzylpenicillin (2.4 grams, 74% yield), which is assayed at approximately 48% pure by the manometric method. - brand name Amcill (Parke-Davis); Omnipen (Wyeth-Ayerst); Polycillin (Apothecon); Principen (Apothecon).
- Therapeutic Function Antibacterial
- General Description Ampicillin was synthesized by Beecham Research Laboratories in 1961 and evaluated for its anti-gram-negative activity by Rolinson et al.. It was the first semisynthetic penicillin showing strong activity against gramnegative bacilli. Although it is hydrolyzed by bacterial penicillinase, it is active against Escherichia coli, Shigella, Proteus mirabilis, and Haemophilus influenzae and is used very widely as an oral antibiotic. Ampicillin also is used by injection for serious infections.
- Contact allergens Ampicillin caused contact dermatitis in a nurse also sensitized to amoxicillin (with tolerance to oral phenoxymethylpenicillin) and in a pharmaceutical factory worker. Systemic drug reactions are common. Crossreactivity is regular with ampicillin and can occur with other penicillins.
- Safety Profile Poison by intracerebral route. Moderately toxic by intraperitoneal route. Human systemic effects by ingestion: fever, agranulocytosis, and other blood effects. An experimental teratogen. Mutation data reported. Questionable carcinogen. When heated to decomposition it emits very toxic fumes of NO, and SOx,.
- Potential Exposure Used as an antibiotic.
-
Veterinary Drugs and Treatments
In dogs and cats, ampicillin is not as well absorbed after oral administration
as amoxicillin and its oral use has largely been supplanted
by amoxicillin. It is used commonly in parenteral
dosage
forms when an aminopenicillin is indicated in all species.
The aminopenicillins, also called the “broad-spectrum” or ampicillin penicillins, have increased activity against many strains of gram-negative aerobes not covered by either the natural penicillins or penicillinase-resistant penicillins, including some strains of E. coli, Klebsiella, and Haemophilus. - First aid If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get
- storage Color Code—Green: General storage may be used.Prior to working with this chemical you should be trained onits proper handling and storage. Store in tightly closedcontainers in a cool, well-ventilated area away from strongoxidizers and moisture.
- Shipping UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
- Incompatibilities May be incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
- Waste Disposal It is inappropriate and possibly dangerous to the environment to dispose of expired or waste pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
Ampicillin Preparation Products And Raw materials
Raw materials
Preparation Products
Global(549)Suppliers
-
Supplier:
Henan Suikang Pharmaceutical Co.,Ltd.
- Tel:+86-18239973690<br/>+86-18239973690
- Email:sales@suikangpharm.com
- Country:China
- ProdList:311
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12813
- Advantage:58
-
Supplier:
Hebei Yanxi Chemical Co., Ltd.
- Tel: +8617531153977
- Email:allison@yan-xi.com
- Country:China
- ProdList:5854
- Advantage:58
-
Supplier:
Hebei Yime New Material Technology Co., Ltd.
- Tel:+86-66697723<br/>+86-17703311139
- Email:admin@china-yime.com
- Country:China
- ProdList:563
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5870
- Advantage:58
-
Supplier:
Hebei ZB Gamay Biological Technology Co.,Ltd
- Tel: +8617330018573
- Email:info@zbvet.net
- Country:China
- ProdList:255
- Advantage:58
-
Supplier:
shandong perfect biotechnology co.ltd
- Tel:+86-53169958659<br/>+86-13153181156
- Email:sales@sdperfect.com
- Country:China
- ProdList:294
- Advantage:58
-
Supplier:
Guangzhou Tengyue Chemical Co., Ltd.
- Tel:+86-86-18148706580<br/>+8618826483838
- Email:evan@tyvovo.com
- Country:China
- ProdList:146
- Advantage:58
-
Supplier:
Wuhan Marco Pharmaceutical Technology Co., Ltd.
- Tel:+86-18572802410<br/>+86-18572802410
- Email:sales@marcopht.com
- Country:China
- ProdList:30
- Advantage:58
Related articles
69-53-4, AmpicillinRelated Search:
- 69-53-4
- Chiral Reagents
- Pharmaceuticals
- Isotope Labeled Compounds
- Intermediates & Fine Chemicals
- veterinary medicine,soluble powder
- Antibacterial Drugs
- Sulfur & Selenium Compounds
- Isotope Labelled Compounds
- Thiazines ,Thiazoles
- API
- 抑制剂
- 农用原粉
- 农用原料
- 其他
- 生物医药-医药原料
- 哌拉西林杂质
- 兽用原料
- 杂质对照品
- 化工
- 医用原料
- 精细化工原料
- 工业化
- 化学原料
- 生物化工
- 标准品
- 医用原料
- 对照品
- 原料中间体-原料药
- 医药化工类
- 对照品-杂质对照品
- 农用化学品原料
- 原料
- 兽药原料
- 化药杂质-氨苄西林
- 哌拉西林优势供应
- 通用生化试剂-抗生素
- 化学试剂
- 饲料添加剂
- 有机化工原料
- 消炎类
- 其它原料及中间体
- 中药对照品
- 医药原材料
- 农药原药
- API原料药-兽药原料药
- 科研试剂咨询13026126800
- 主打
- 其他原料
- 医药兽药
- 医药原料药
- 食品添加剂
- 小分子抑制剂
- 稳定同位素标记化合物
- 医药原料
- 维生素与氨基酸
- 同位素标记物
- 青霉素类