ChemicalBook > CAS DataBase List > APALCILLIN
APALCILLIN
APALCILLIN
- CAS No.63469-19-2
- Chemical Name:APALCILLIN
- CBNumber:CB7151479
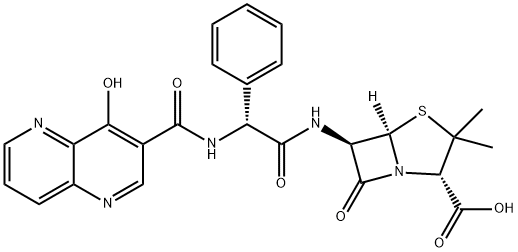
- Molecular Formula:C25H23N5O6S
- Formula Weight:521.54
- MOL File:63469-19-2.mol
APALCILLIN Property
- FDA UNII 3373RT9U7A
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
APALCILLIN Chemical Properties,Usage,Production
- Originator Lumota,Thomae,W. Germany,1982
- Uses Antibacterial.
- Definition ChEBI: Apalcillin is a penicillin and a 1,5-naphthyridine derivative. It is a conjugate acid of an apalcillin(1-).
-
Manufacturing Process
(a) Preparation of 6-D-α-aminobenzylpenicillin phenacyl ester: To a
suspension of phenacyl 6-aminopenicillanate hydrochloride (1.85 g) and Dphenylglycyl
chloride hydrochloride (1.29 g) in dichloromethane (20 ml),
sodium bicarbonate (1.05 g) was added, and the resultant mixture was stirred while cooling with ice for 6 hours. The reaction mixture was filtered to
eliminate the by-produced sodium chloride. The filtrate was admixed with
isopropanol and concentrated under reduced pressure by the aid of a rotary
evaporator. After the evaporation of dichloromethane, the precipitate was
collected by filtration to give the objective compound in the form of the
hydrochloride (2.19 g) MP 142° to 148°C (decomposition).
(b) Preparation of D-α-(4-hydroxy-1,5-naphthyridine-3- carbonamido)benzylpenicillin: To a solution of 6-D-α-aminobenzylpenicillin phenacyl ester (hydrochloride) (2.01 g) and triethylamine (0.808 g) in dimethylformamide (20 ml), 4-hydroxy-1,5-naphthyridine-3-carboxylic acid Nsuccinimide ester [MP 310° to 311°C (decomposition)] (1.15 g) was added while cooling with ice, and the resultant mixture was stirred for 1 hour. Stirring was further continued at room temperature for 2 hours. After cooling with ice, 1% sodium bicarbonate solution (100 ml) was added thereto. The precipitated crystals were collected by filtration, washed with water and dried over phosphorus pentoxide to give D-(α-4-hydroxy-1,5-naphthyridine-3- carboxamido)benzylpenicillin phenacyl ester (2.17 g).
The above product was dissolved in dimethylformamide (65 ml), sodium thiophenoxide (0.89 g) was added thereto, and the resultant mixture was stirred at room temperature for 1 hour. To the resultant mixture, acetone (650 ml) was added, and the separated crystals were collected by filtration and washed with acetone and ether in order to give the objective compound in the form of the sodium salt (1.3 g).
In the above procedure, the use of 4-hydroxy-1,5-naphthyridine-3-carbonyl chloride in place of 4-hydroxy-1,5-naphthyridine-3-carboxylic acid Nsuccinimide ester can also afford the same objective compound as above. The use of sodium thio-n-propoxide in place of sodium thiophenoxide can also give the objective compound in the form of the sodium salt. - Therapeutic Function Antibacterial
- Antimicrobial activity A semisynthetic acylaminopenicillin supplied as the sodium salt for parenteral administration. The antibacterial spectrum and toxicity profile are similar to those of the acylureidopenicillins. It is relatively labile to many β-lactamases, including the common TEM plasmid-mediated enzyme. It has very limited commercial availability.
APALCILLIN Preparation Products And Raw materials
Raw materials
Preparation Products
Global(9)Suppliers
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-89586680<br/>+86-18192503167
- Email:1026@dideu.com
- Country:China
- ProdList:7863
- Advantage:58
- Supplier: Beijing HuaMeiHuLiBiological Chemical
- Tel:010-56205725
- Email:waley188@sohu.com
- Country:China
- ProdList:12335
- Advantage:58
63469-19-2, APALCILLINRelated Search:
- C25H23N5O6S
- 63469-19-2
- APALCILLIN USP/EP/BP
- 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[(2R)-2-[[(4-hydroxy-1,5-naphthyridin-3-yl)carbonyl]amino]-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-, (2S,5R,6R)-
- Lumota
- Apalcilline
- Apalcilina [inn-spanish]
- Apalcilina
- 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 6-(((2R)-(((4-hydroxy-1,5-naphthyridin-3-yl)carbonyl)amino)phenylacetyl)amino)-3,3-dimethyl-7-oxo-, (2S,5R,6R)-
- 4-Thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid, 6-(((((4-hydroxy-1,5-naphthyridin-3-yl)carbonyl)amino)phenylacetyl)amino)-3,3-dimethyl-7-oxo-, (2S-(2-alpha,5-alpha,6-beta(S*)))-
- (6R)-6-((R)-2-(4-Hydroxy-1,5-naphthyridin-3-carboxamido)-2-phenylacetamido)penicillansaeure
- (2S,5R,6R)-6-((R)-2-(4-Hydroxy-1,5-naphthyridin-3-carboxamido)-2-phenylacetamido)-3,3-dimethyl-7-oxo-5-thia-1-azabicyclo(3.2.0)heptan-2-carboxylic acid
- (2S,5R,6R)-6-((R)-2-(4-Hydroxy-1,5-naphthyridin-3-carboxamido)-2-phenylacetamido)-3,3-dimethyl-7-oxo-5-thia-1-azabicyclo(3.2.0)heptan-2-carbonsaeure
- 6α-[(R)-2-[(4-Hydroxy-1,5-naphthyridin-3-yl)carbonylamino]-2-phenylacetylamino]penicillanic acid
- (2S,5R,6R)-6α-[[(R)-(4-Hydroxypyrido[3,2-b]pyridin-3-ylcarbonylamino)phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2β-carboxylic acid
- APALCILLIN
- 5-alpha,6-beta(s*)))-ph
- 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylicacid,6-(((((4-hydroxy-1,5-napht
- 3-dimethyl-7-oxo-hyridin-3-yl)carbonyl)amino)phenylacetyl)amino)-(2s-(2-al