ChemicalBook > CAS DataBase List > BACAMPICILLIN
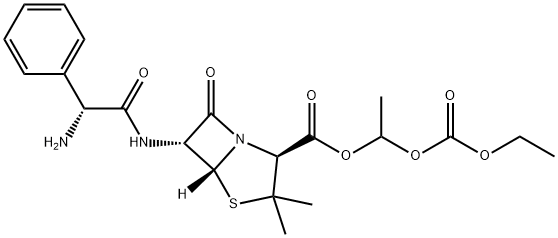
BACAMPICILLIN
BACAMPICILLIN
- CAS No.50972-17-3
- Chemical Name:BACAMPICILLIN
- CBNumber:CB1343108
- Molecular Formula:C21H27N3O7S
- Formula Weight:465.52
- MOL File:50972-17-3.mol
BACAMPICILLIN Property
- Boiling point 678.4±55.0 °C(Predicted)
- Density 1.37±0.1 g/cm3(Predicted)
- storage temp. Store at -20°C
- solubility Soluble in DMSO
- pka pKa 6.8 (Uncertain)
- CAS DataBase Reference 50972-17-3
- FDA UNII 8GM2J22278
- ATC code J01CA06
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
BACAMPICILLIN Chemical Properties,Usage,Production
- Originator Penglobe,Astra,W. Germany,1977
- Definition ChEBI: A penicillanic acid ester that is the 1-ethoxycarbonyloxyethyl ester of ampicillin. It is a semi-synthetic, microbiologically inactive prodrug of ampicillin.
-
Manufacturing Process
1'-Ethoxycarbonyloxyethyl 6-(D-α-azidophenylacetamido)penicillinate (98 g)
was prepared from sodium 6-(D-α-azidophenylacetamido)penicillinate (397 g,
1 mol), α-chlorodiethylcarbonate (458 g, 3 mols) and sodium bicarbonate
(504 g, 6 mols). The product showed strong IR absorption at 2090 cm-1 and
1780-1750 cm-1 showing the presence of azido group and β-lactam and ester
carbonyls.
It was dissolved in ethyl acetate (700 ml) and hydrogenated at ambient conditions over a palladium (5%)on carbon catalyst (18 g). The catalyst was removed by filtration and washed with ethyl acetate. The combined filtrates were extracted with water at pH 2.5 by addition of dilute hydrochloric acid. Lyophilization of the aqueous phase gave the hydrochloride of 1'- ethoxycarbonyloxyethyl 6-(D-α-aminophenylacetarnido)penicillinate (94 g), MP 171°-176°C. - Therapeutic Function Antibacterial
- Metabolism Although comparatively well absorbed (30–55%), the oral efficacy of ampicillin for systemic infections can be enhanced significantly through the preparation of pro-drugs. In contrast to ampicillin itself, which is amphoteric, bacampicillin is a weak base and is very well absorbed in the duodenum (80–98%). Enzymatic ester hydrolysis in the gut wall liberates carbon dioxide and ethanol, followed by spontaneous loss of acetaldehyde and production of ampicillin. The acetaldehyde is metabolized oxidatively by alcohol dehydrogenase to produce acetic acid, which joins the normal metabolic pool.
BACAMPICILLIN Preparation Products And Raw materials
Raw materials
Preparation Products
Global(33)Suppliers
-
Supplier:
BOC Sciences
- Tel:+1-631-485-4226
- Email:inquiry@bocsci.com
- Country:United States
- ProdList:19552
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Career Henan Chemica Co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:laboratory@coreychem.com
- Country:China
- ProdList:30231
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-89586680<br/>+86-18192503167
- Email:1026@dideu.com
- Country:China
- ProdList:7863
- Advantage:58
-
Supplier:
DAYANG CHEM (HANGZHOU) CO.,LTD
- Tel: 13515810918
- Email:thtang@dycnchem.com
- Country:China
- ProdList:53881
- Advantage:58
-
Supplier:
Amadis Chemical Company Limited
- Tel:571-89925085
- Email:sales@amadischem.com
- Country:China
- ProdList:131957
- Advantage:58
- Supplier: Beijing HuaMeiHuLiBiological Chemical
- Tel:010-56205725
- Email:waley188@sohu.com
- Country:China
- ProdList:12335
- Advantage:58
- Supplier: MedChemexpress LLC
- Tel:021-58955995
- Email:sales@medchemexpress.cn
- Country:United States
- ProdList:4861
- Advantage:58
- Supplier: Musechem
- Tel:+1-800-259-7612
- Email:info@musechem.com
- Country:United States
- ProdList:4660
- Advantage:60
- Supplier: Shanghai Han-Xiang Chemical Co., Ltd.
- Tel: 15971444841
- Email:amber@biochempartner.com
- Country:China
- ProdList:3061
- Advantage:58
50972-17-3, BACAMPICILLINRelated Search:
- PIVAMPICILLIN BACAMPICILLIN HYDROCHLORIDE,Bacampicillin HCl,BACAMPICILLIN HYDROCHLORIDE USP STANDARD,BACAMPICILLIN HYDROCHLORIDE EP STANDARD (S)-Bacampicillin hydrochloride S-Bacampicillin R-Bacampicillin (R)-Bacampicillin hydrochloride BACAMPICILLIN HYDROCHLORIDE EPB(CRM STANDARD) Ampicillin ETHYL 3-(METHYLTHIO)BUTYRATE 4-METHOXY-2-METHYL-2-BUTANETHIOL BACAMPICILLIN TRIMETHYLAMMONIUM FORMIATE
- 医药、农药及染料中间体
- 医药原料药
- C21H27N3O7S
- 化合物 T10448
- 巴氨西林
- 50972-17-3
- BACAMPICILLIN USP/EP/BP
- 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[(2R)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-, 1-[(ethoxycarbonyl)oxy]ethyl ester, (2S,5R,6R)-
- (2S,5R,6R)-6-[[(2R)-2-amino-1-oxo-2-phenylethyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 1-ethoxycarbonyloxyethyl ester
- 6α-[[(R)-2-Amino-2-phenylacetyl]amino]penicillanic acid 1-[(ethoxycarbonyl)oxy]ethyl ester
- (2S,5R,6R)-6-[[(2R)-2-Amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 1-(ethoxycarbonyloxy)ethyl ester
- Bacampicillin (base and/or unspecified salts)
- Carampicillin
- 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[(2R)-aminophenylacetyl]amino]-3,3-dimethyl-7-oxo-, 1-[(ethoxycarbonyl)oxy]ethyl ester, (2S,5R,6R)-
- 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[(aminophenylacetyl)amino]-3,3-dimethyl-7-oxo-, 1-[(ethoxycarbonyl)oxy]ethyl ester, [2S-[2α,5α,6β(S*)]]-
- BACAMPICILLIN HCL USP(CRM STANDARD)
- BACAMPICILLIN