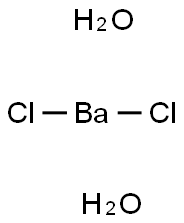ChemicalBook > CAS DataBase List > Barium chloride dihydrate
Barium chloride dihydrate
Barium chloride dihydrate
- CAS No.10326-27-9
- Chemical Name:Barium chloride dihydrate
- CBNumber:CB3204825
- Molecular Formula:BaCl2H4O2
- Formula Weight:244.26
- MOL File:10326-27-9.mol
Barium chloride dihydrate Property
- Melting point 962 °C
- Boiling point 1560 °C
- bulk density 1200-1400kg/m3
- Density 3.86
- storage temp. Store at +5°C to +30°C.
- solubility Water (Slightly)
- form Solid
- Specific Gravity 3.097
- color White
- PH 5.2-8.2 (25℃, 50mg/mL in H2O)
- Water Solubility soluble
- Merck 14,971
- Exposure limits ACGIH: TWA 0.5 mg/m3
NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 - Stability Stable. Incompatible with interhalogens, furan-2-percarbonic acid.
- CAS DataBase Reference 10326-27-9(CAS DataBase Reference)
- FDA UNII EL5GJ3U77E
- EPA Substance Registry System Barium chloride dihydrate (10326-27-9)
- UNSPSC Code 12352302
- NACRES NB.24
Safety
-
Symbol(GHS)

- Signal wordDanger
- Hazard statements H301-H319-H332
- Precautionary statements P261-P264-P270-P301+P310-P304+P340+P312-P305+P351+P338
Barium chloride dihydrate Price
More Price(27)
- Brand: Sigma-Aldrich(India)
- Product number: B0750
- Product name : Barium chloride dihydrate
- Purity: ≥99%
- Packaging: 500G
- Price: ₹3355.75
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: B0750
- Product name : Barium chloride dihydrate
- Purity: ≥99%
- Packaging: 1KG
- Price: ₹4827.95
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: B0750
- Product name : Barium chloride dihydrate
- Purity: ≥99%
- Packaging: 100G
- Price: ₹5737.25
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 217565
- Product name : Barium chloride dihydrate
- Purity: ACS reagent, ≥99%
- Packaging: 500G
- Price: ₹2468.1
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich
- Product number: 217565
- Product name : Barium chloride dihydrate
- Purity: ACS reagent, ≥99%
- Packaging: 100G
- Price: ₹4708.88
- Updated: 2022/06/14
- Buy: Buy
Barium chloride dihydrate Chemical Properties,Usage,Production
- Description Barium chloride dihydrate is recrystallized from the water solutions of barium chloride. Barium chloride is used for the test of sulfate ions, for making pigment, for the purification of brine solution, in the manufacture of heat treating bath, and in the fireworks for green color. Barium Chloride is also used to set up porcelain enamels for sheet steel and to produce blanc fixe. Together with various matrices, barium chloride is used to fabricate novel ammonia sorbents for adsorptive heat transformation. Furthermore, barium chloride is used to modify the microstructure of polyvinyl alcohol (PVA) polymer films which have rich variety of applications.
-
References
[1] J.V. Veselovskaya, M.M. Tokarev and Yu. I. Aristov, Novel ammonia sorbents ‘‘porous matrix modified by active salt” for adsorptive heat transformation 1. Barium chloride in various matrices, Applied Thermal Engineering, 2010, vol. 30, 584-589
[2] R.F. Bhajantri, V. Ravindrachary, A. Harisha, C. Ranganathaiah and G.N. Kumaraswamy, Effect of barium chloride doping on PVA microstructure: positron annihilation study, Applied Physics A, 2007, vol. 87, 797-805
[3] Daniel C. Hall and Edward F. Steinbring, Process of making pigment barium sulphate from barium chloride, Patent, 1935
[4] Paul H. Ralston and Charles T. Roland, Method of preparing sodium chloride brine of high purity, Patent, 1954
[5] http://earthsky.org
[6] Alber Erdmann and Elli Boelke, Salt bath for heat treating carbon alloyed steel, Patent, 1954 - Description Barium chloride is a white/colourless solid and stable under ordinary conditions of use and storage. It is incompatible with bromine trifluoride and 2-furan percarboxylic acid (anhydrous).
- Chemical Properties White solid
- Chemical Properties Barium chloride is a white/colorless solid, stable under ordinary conditions of use and storage. It is incompat ible with bromine tril uoride, 2-furan percarboxylic acid (anhydrous).
- Uses Barium chloride dihydrate is mainly used in the purification of brine solution in caustic chlorine plants, as a reagent in the test for sulfate ion in chemical lab, and as a cleansing agent in the production of other barium chemicals and lubricating oil additives. It is used in the production of molecular sieves, pigments, and paper coatings, and serves as a precursor to fabricate the doped barium zirconate.
- Definition ChEBI: A hydrate that is the dihydrate form of barium chloride.
- General Description White crystalline solid with a bitter, salty taste.
- Air & Water Reactions Water soluble.
- Reactivity Profile Barium chloride dihydrate may react violently with BrF3 and 2-furan percarboxylic acid in its anhydrous form.
- Health Hazard Exposures to barium chloride cause sore throat, coughing, and labored breathing, and become harmful and fatal if swallowed or inhaled. Prolonged exposures cause irritation to the skin, eyes, and respiratory tract, and involve the heart, respiratory system, and the CNS. An accidental ingestion of barium chloride causes severe gastroenteritis, abdomi- nal pain, vomiting, diarrhea, tremors, faintness, paralysis of arms and legs, and a slow or irregular heartbeat. In severe cases, barium chloride may cause collapse and death from respiratory failure.
- Fire Hazard Flash point data for Barium chloride dihydrate are not available. Barium chloride dihydrate is probably combustible.
- Safety Profile A poison by intraperitoneal route.When heated to decomposition it emits toxic vapors of Baand Cl-.
- storage Barium chloride should be kept stored in a tightly closed container. Protect from physi- cal damage. Store in a cool, dry, ventilated area away from sources of heat, moisture, and incompatibilities. Containers of this material may be hazardous when empty since they retain product residues (dust, solids); observe all warnings and precautions listed for the product.
- Purification Methods It is crystallised twice from water (2mL/g) and dried in an oven to constant weight. The solubilities of the hydrate (% of anhydrous wt) in H2O are 31.6 at 0o, 35.7 at 20o and 58.7 at 100o.
- Precautions Occupational workers should be careful during handling of barium hydroxide. Workers should wear impervious protective clothing, including boots, gloves, laboratory coat, apron, or coveralls, as appropriate, to prevent skin contact. Workers should use chemical safety goggles or full-face shields. The workplace should maintain an eye-wash fountain and quick-drench facilities.
Barium chloride dihydrate Preparation Products And Raw materials
Raw materials
Preparation Products
Global(376)Suppliers
-
Supplier:
Hebei Andu Technology Com.,Ltd
- Tel:+86-17798073498<br/>+86-17798073498
- Email:admin@hbandu.com
- Country:China
- ProdList:378
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5870
- Advantage:58
-
Supplier:
Shandong Juchuang Chemical Co., LTD
- Tel: +16837774622
- Email:admin@juchuangchem.com
- Country:China
- ProdList:295
- Advantage:58
-
Supplier:
Hebei Zhuanglai Chemical Trading Co.,Ltd
- Tel: +8613343047651
- Email:admin@zlchemi.com
- Country:China
- ProdList:3692
- Advantage:58
-
Supplier:
HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
- Tel: +8615350851019
- Email:admin@86-ss.com
- Country:China
- ProdList:1001
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418679<br/>+8618949832763
- Email:info@tnjchem.com
- Country:China
- ProdList:2986
- Advantage:55
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29860
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
Related articles
10326-27-9, Barium chloride dihydrateRelated Search:
- Copper(II) chloride dihydrate BARIUM PERCHLORATE Barium fluoride BARIUM NITRITE Barium iodide Barium carbonate Barium nitrate BARIUM HYDRIDE Barium peroxide Barium oxide Barium selenite BARIUM BROMIDE Barite Barium manganate Barium titanate BARIUM CHLORATE MONOHYDRATE Barium chloride Barium hydroxide
- Inorganic Chemicals
- Puriss p.a. ACS
- Analytical Reagents for General Use
- A-B, Puriss p.a. ACSSynthetic Reagents
- Synthetic Reagents
- Inorganic Salts
- Barium
- Routine Reagents
- Essential Chemicals
- ACS GradeSynthetic Reagents
- metal halide
- Salts
- BariumMetal and Ceramic Science
- Barium Salts
- A-B, Puriss p.a. ACS
- 通用试剂-钡类
- 日用化工
- 醇类
- 其他化工产品
- 高端化学
- 电池材料
- 其他行业应用化学品
- 有机硅产品
- 其他危险化学品
- 有机类
- 无机产品
- 有机产品
- 原料药
- 精细化工品
- 无机原料
- 化工原料
- 氯化物
- 无机化工原料
- 化工材料
- 化工产品
- 二水氯化钡
- 合成材料中间体
- 生化试剂
- 助剂
- 化工产品-有机化工
- 化工
- 氯化物
- 无机盐
- 无机化工产品
- Ca钡化合物
- 催化和无机化学
- 有机硼化合物
- 标准品和标准物质
- 无机化合物和盐
- 钡
- 通用试剂
- 轻金属
- H4BaCl2O2
- H4BaClO2
- BaCl
- BaCl22H2O
- BaCl32H2O
- BaCl22H20