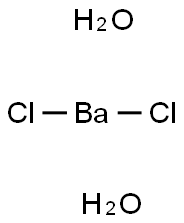Barium chloride dihydrate: uses and Synthesis method
Introduction
Barium chloride dihydrate is the dihydrate form of barium chloride. It is a white crystalline solid with a bitter, salty taste and a role as a potassium channel blocker.

Synthesis method
Barium chloride dihydrate is prepared by reacting aqueous barium sulfide with hydrochloric acid. The reaction mixture is purified and then evaporated to yield crystalline barium chloride dihydrate.
Barium chloride dihydrate is also made by the neutralization of barium hydroxide with HCl in water. The product crystallizes as the dihydrate BaCl2.2H2O. If this heated, dehydration to anhydrous BaCl2 occurs.
Uses
Barium Chloride Dihydrate is used in wastewater treatment, the production of PVC stabilizers, oil lubricants, barium chromate, and barium fluoride. It is also used in pigments, aluminum refining, leather tanning and colouring, the manufacture of magnesium metal, ceramics, glass, and paper products, as a pesticide, and in medicine, as a cardiac stimulant. As an inexpensive, soluble barium salt, barium chloride is widely applied in the laboratory. It is commonly used as a test for sulfate ions.
Hazardous
Typical symptoms of acute exposures:
Eye irritation, tearing pain. Gastroenteritis, nausea, vomiting, muscle spasm, slow pulse. Tingling sensation in the extremities. Cardiac arrhythmia, paralysis, possible death.
Principal target organ(s) or system(s):
Respiratory system, heart, central nervous system, kidneys.
Barium chloride can be absorbed into the body by inhalation and ingestion. It is a mild skin irritant. The barium ion is considered a muscle poison; via the central nervous system, it stimulates affected muscles and causes paralysis. The symptoms of barium poisoning are attributed to a barium ion-induced hypokalemia, probably due to a transfer of potassium from extracellular to intracellular components.
[1] Indhold (mst.dk) https://ntp.niehs.nih.gov/sites/default/files/ntp/htdocs/lt_rpts/tr432.pdf
[2] Young, Jay A. “Chemical Laboratory Information Profile: Barium Chloride Dihydrate.” Journal of Chemical Education 79 5 (2002): 554.
References:
[1] YOUNG J A. Chemical Laboratory Information Profile: Barium Chloride Dihydrate[J]. Journal of Chemical Education, 2002, 79 5: 535-640. DOI:10.1021/ed079p554.You may like
Lastest Price from Barium chloride dihydrate manufacturers

US $999.00-666.00/ton2025-04-21
- CAS:
- 10326-27-9
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 5000

US $3.60/kg2025-04-18
- CAS:
- 10326-27-9
- Min. Order:
- 1kg
- Purity:
- ≥99%
- Supply Ability:
- 3000tons/month