Recent research advances of tin(II) fluoride
Introduction
Tin(II) fluoride (Stannous fluoride;SnF2;Figure 1) dissolves in water, methanol, and in hot dmso, but is insoluble in, and does not form isolable adducts with, thf, MeCN, 1,2-dimethoxyethane or 1,4-dioxane. This contrasts with the formation of [SnF4(L)2] (L = thf or MeCN), which are useful molecular synthons for many complexes of SnF4. SnF2 is moderately soluble in MeOH to give a colourless solution with a sharp F-NMR resonance at δ = −98.4, but no solid complex could be isolated on removal of the solvent. [1]Tin(II) fluoride, which unlike sodium fluoride can be used in combination with calcium-based abrasives, has been incorporated in dentifrices since the 1950s to provide protection against caries, pathogenic bacteria, gingivitis, hypersensitivity, and the development of plaque. There is considerable evidence for its efficacy as a therapeutic agent with a wide spectrum of beneficial properties. However, its clinical usage was limited because of as tringent taste, and in some patients, its use resulted in extrinsic staining of the teeth. Tin(II) fluoride was also somewhat unstable in aqueous solution. The latter problem was resolved with the introduction of stabilized tin(II) fluoride in the 1990s, which rendered more available tin(II) fluoride and resulted in a renewed interest in the wide range of benefits offered by tin(II) fluoride in dentifrices.[2]

Application research of tin(II) fluoride
1.Recent advances in tin(II) fluoride technology: antibacterial efficacy and mechanism of action towards hypersensitivity.
Tin(II) fluoride(Stannous fluoride;SnF2) is highly susceptible to oxidation and hydrolysis but both anhydrous and aqueous preparations can be well established by proper formulation. When stability in aqueous preparations is achieved by the use of certain strong complexing agents, reduced antibacterial activity is observed which may be attributed to reduced bioavailability of the stannous ion. In contrast, an anhydrous SnF2 preparation maintains stannous ion in a stable but, uncomplexed form. This preparation displays antibacterial activity in saliva and delivers stannous ion which is absorbed onto surfaces making them less susceptible to plaque formation for an extended period of time (hours). When this anhydrous preparation is brushed onto dentine in vitro or in situ, one observes a nearly complete coverage of the dentine surface and occlusion of tubules by a tin-rich surface deposit. This finding indicates that the observed clinical efficacy of this preparation at relieving hypersensitivity is due to occlusion of tubules by a mixture of low solubility complexes of tin. A water-based tin(II) fluoride preparation containing strongly complexed stannous ions does not form a surface coating on dentine in vitro suggesting that this preparation may not be optimal for treating hypersensitivity. Overall, the findings indicate that the stannous ions in a tin(II) fluoride preparation must be maintained in a stable, bioavailable form for optimal efficacy against plaque and hypersensitivity to be obtained. The results suggest that these properties are provided by stable anhydrous preparations but are difficult to achieve simultaneously in aqueous preparations. When properly formulated,tin(II) fluoride preparations can provide multiple oral therapeutic benefits.[3]
2.Tin(II) fluoride vs. tin(II) chloride--a comparison of their coordination chemistry with neutral ligands.
Reaction of SnF2 in MeOH with the appropriate neutral N- or O-donor ligands produces [SnF(2,2’- bipy)]2SnF6, [SnF(1,10-phen)]2SnF4 and [SnF2(L)] L = Me3PO, dmso or pyNO). The X-ray structures of [SnF- (2,2’-bipy)]2SnF6, [SnF(1,10-phen)]2SnF4 and [SnF2(dmso)], reveal trigonal pyramidal Sn(II) cores with longer fluorine bridges completing distorted 5- or 6-coordination. Attempts to prepare SnF2 adducts with various phosphine or diphosphine ligands in MeCN failed, whilst in CH2Cl2 solution complex reactions involving the solvent occurred. The NHC, 1,3-(2,6-di-isopropylphenyl)imidazol-2-ylidene (IDiPP) and SnF2 produced the imidazolium salt, [IDiPPH]SnF3, the crystal structure of which revealed the first example of a discrete trifluorostannate(II) ion. In contrast, diphosphine complexes of tin(II) chloride formed readily, including [SnCl2{Me2P(CH2)2PMe2}], [SnCl2{o-C6H4(PMe2)2}], [SnCl2{o-C6H4(PPh2)2}] and [(SnCl2)2(μ-Ph2P(CH2)2PPh2)], which were characterised by X-ray crystallography. The structures of [SnCl2{Me2P(CH2)2PMe2}] and [SnCl2{o-C6H4(PMe2)2}] reveal chloride-bridged dimers, but [SnCl2{o-C6H4-(PPh2)2}], although also dimeric, has very asymmetric diphosphine coordination best described as κ1 . The structures of [(SnCl2)2(μ-Ph2P(CH2)2PPh2)] and of [SnCl{o-C6H4(AsMe2)2}]SnCl3 reveal trigonal pyramidal cores, but with longer Sn⋯Cl bridges affording polymeric structures. The synthesis of [SnCl2(R3EO)2] (R = Ph, E = P or As; and R = Me, E = P) are also reported, along with the structure of [SnCl2(Me3PO)2], which contains distorted tetragonal pyramidal Sn(II) coordination. X-ray structures are also reported for [(PMe3)2CH2][SnCl3]2 and [Ph2P(H)(CH2)2P(H)Ph2][SnCl3]2, obtained as by-products from the attempts to synthesise phosphine complexes, as well as [(o-C6H4(PMe2)2CH2]I2. All complexes were characterised by microanalysis, IR and multinuclear NMR spectroscopy ( 1H, 19F{ 1H}, 31P{ 1H } and, where solubility allowed, 119 Sn). Comparisons are drawn with corresponding Sn(IV) and Ge(II) complexes.[1]
3.Lipopolysaccharide and Lipoteichoic Acid Virulence Deactivation by Tin(II) fluoride.
Oral bacterial pathogens promote gingivitis and periodontal disease. Bacterial endotoxins, also known as lipopolysaccharides (LPSs) and lipoteichoic acids (LTAs), are known to enhance bacterial pathogenicity through binding with Toll-like receptors (TLRs), a group of pattern recognition receptors critical to the activation of innate immunity, that are expressed on host cells. Both LPS and LTA contain lipophilic domains and anionic charges that may be susceptible to reactivity with tin(II) fluoride, a commonly used ingredient clinically proven for the treatment and prevention of gingivitis. This study examined the effects of tin(II) fluoride on Toll-like receptor activation in response to bacterially derived LPS and LTA in select cell lines and secretion of inflammatory cytokines from human primary peripheral monocytes likewise exposed to LPS.
TLR4 and TLR2 transfected HEK293 cells and THP1-Dual™ cells were exposed to bacterial LPS and LTA in the presence of increasing concentrations of tin(II) fluoride. Gene expression was assessed by measurement of secreted embryonic alkaline phosphatase (SEAP) reporter gene for HEK293 cells and SEAP and luciferase for THP-1 cells. Cell viability was confirmed using PrestoBlue. Human primary monocytes were treated with LPS with various concentrations of supplemented tin(II) fluoride, and cytokine expression was directly measured.
Tin(II) fluoride inhibited gene expression response of TLR4 and TLR2 in HEK293 cells and THP1-Dual™ cells in a dose response fashion, producing complete inhibition at micromolar concentrations. The addition of tin(II) fluoride suppressed production of TNF-a, IFN-g, IL12p70, IL10, IL-1b, IL2, and IL-6, and also increased secretion of Il-8 in dose response fashion. Viability assays confirmed no effects of LPS or tin(II) fluoride on viability of HEK293, THP-1, and primary human monocytes.
These results support the potential for tin(II) fluoride to provide clinical gingivitis benefits by directly decreasing the pathogenicity of plaque biofilms by blocking reactivity of LPS and LTA ligands with tissue receptors associated with inflammation. These learnings may influence recommendations for patients at risk for plaque-related diseases.[4]
References
[1]Gurnani C, Hector AL, Jager E, Levason W, Pugh D, Reid G. Tin(II) fluoride vs. tin(II) chloride--a comparison of their coordination chemistry with neutral ligands. Dalton Trans. 2013;42(23):8364-8374. doi:10.1039/c3dt50743b
[2]Sensabaugh C, Sagel ME. Stannous fluoride dentifrice with sodium hexametaphosphate: review of laboratory, clinical and practice-based data. J Dent Hyg. 2009;83(2):70-78.
[3]Miller S, Truong T, Heu R, Stranick M, Bouchard D, Gaffar A. Recent advances in stannous fluoride technology: antibacterial efficacy and mechanism of action towards hypersensitivity. Int Dent J. 1994;44(1 Suppl 1):83-98.
[4]Haught C, Xie S, Circello B, et al. Lipopolysaccharide and Lipoteichoic Acid Virulence Deactivation by Stannous Fluoride. J Clin Dent. 2016;27(3):84-89.
You may like
Lastest Price from Tin(II) fluoride manufacturers

US $0.00-0.00/KG2025-04-15
- CAS:
- 7783-47-3
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg

US $55.00/kg2025-04-15
- CAS:
- 7783-47-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000kg/week
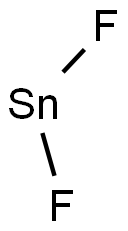

![3345-29-7 1,1,2,2,9,9,10,10-Octafluoro[2.2]paracyclophanesynthesisuses](/NewsImg/2025-04-18/6388058708467047983386103.png)