Description
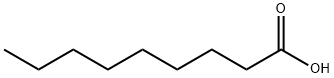
Nonanoic acid (also known as pelargonic acid; chemical formula: CH3
(CH2)7COOH) is a kind of organic carboxylic acid compound. It is
naturally existed in the oil of pelargonium in the form of esters.
It is commonly used in conjunction with glyphosate which is a kind
of non-selective herbicide in order to obtain a quick burn-down
effect in the control of weeds in turfgrass. It is also a potent
antifungal agent which can inhibit spore germination and mycelial
growth of pathogenic fungi. Its synthetic esters, such as methyl
nonanoate can be used as flavorings. Moreover, it can also be used
in the preparation of plasticizers and lacquers. It can also be
potentially used for the treatment of seizures.
References
https://en.wikipedia.org/wiki/Nonanoic_acid
http://www.abcam.com/nonanoic-acid-pelargonic-acid-ab143891.html
Description
Nonanoic acid , also called pelargonic acid, is an organic compound composed of a nine - carbon chain terminating in a carboxylic acid with structural formula CH
3(CH
2)
7COOH. Nonanoic acid forms esters—nonanoates. It is a clear, oily liquid with an unpleasant, rancid odor. It is nearly insoluble in water, but very soluble in chloroform, ether, and hexane.
Its refractive index is 1.4322. Its critical point is at 712 K ( 439 °C ) and 2.35 MPa.
Chemical Properties
Colorless or yellowish, combustible, oily
liquid. Faint odor.
Chemical Properties
clear colorless liquid
Chemical Properties
Nonanoic acid has a fatty, characteristic odor and a corresponding unpleasant taste. This compound is also reported as
having a cheese, waxy flavor
Chemical Properties
Nonanoic acid has a fatty, characteristic odor and a corresponding unpleasant taste. May be prepared by oxidation of methylnonyl ketone; by oxidation of oleic acid; or from heptyl iodide via malonic ester synthesis.
Originator
Pellar,Crookes Barnes, US ,1960
Occurrence
Nonanoic acid is a fatty acid which occurs naturally as esters in the oil of pelargonium. Synthetic esters, such as methyl nonanoate, are used as flavorings.
Nonanoic acid is also used in the preparation of plasticizers and lacquers.
The derivative 4-nonanoylmorpholine is an ingredient in some pepper sprays.
The ammonium salt of nonanoic acid, ammonium nonanoate, is used as an herbicide.
Uses
The primary uses of this acid are in organic synthesis and in
the manufacture of lacquers, plastics, pharmaceuticals, synthetic
odors and flavorings, gasoline additives, flotation
agents, lubricants, and vinyl plasticizers. Nonanoic acid
has found some use in pharmaceutical preparations and as
a topical bactericide and fungicide. It is also used in
herbicides.
Uses
In the production of hydrotropic salts (hydrotropic salts form aqueous solutions which dissolve sparingly soluble substances to a greater extent than water); in the manufacture of lacquers, plastics.
Uses
Intermediates of Liquid Crystals
Definition
ChEBI: A C9 straight-chain saturated fatty acid which occurs naturally as esters of the oil of pelargonium. Has antifungal properties, and is also used as a herbicide as well as in the preparation of plasticisers and lacquers.
Preparation
Nonanoic acid is produced by malonic ester synthesis, from Heptyl iodide.
Manufacturing Process
A body of liquid, 18 inches high, comprising a 35% (by weight) solution of technical (95%) oleic acid in n-propanol, is maintained at a temperature of 86°C in a reactor. The solution also contains dissolved therein 0.042% by weight of cobalt, in the form of cobalt naphthenate. From the bottom of the reactor very fine bubbles of air are passed into and through the solution at the rate of about 0.3 cubic feet per minute, measured at standard conditions, per square foot for 72 hours. The gases leaving the reactor are first passed through an ice water reflux condenser and then vented to the atmosphere. At the end of the 72 hour period the reaction mixture is separated into its components. It is found that 60% of the oleic acid has been consumed in the reaction. For each pound of oleic acid consumed there are obtained 0.30 pound of azelaic acid (representing an efficiency of 46%, calculated on the basis that the technical oleic acid is 100% oleic acid), 0.13 pound of pelargonic acid (representing an efficiency of 23%) and 0.21 pound of 9,10dihydroxystearic acid (representing an efficiency of 19%).
Therapeutic Function
Fungicide
Aroma threshold values
Detection: 3 to 9 ppm
Taste threshold values
Taste characteristics at 10 ppm: fatty, waxy, and cheesy with a mild, sweet, creamy background
Synthesis Reference(s)
Journal of the American Chemical Society, 93, p. 195, 1971
DOI: 10.1021/ja00730a033Organic Syntheses, Coll. Vol. 2, p. 474, 1943
Tetrahedron Letters, 9, p. 5689, 1968
General Description
Nonanoic acid occurs naturally in Pelargonium L′Her. It one of the flavor constituents of cooked rice, acerola fruit, licorice and yogurt.
Agricultural Uses
Herbicide, Fungicide: Pelargonic acid occurs naturally in many plants and
animals. It is used to control the growth of weeds and as a
blossom thinner for apple and pear trees. It is also used as
a food additive; as an ingredient in solutions used to commercially
peel fruits and vegetables
Trade name
CIRRASOL®-185A; ECONOSAN®;
EMERY® 202 (mixture with n-octoic acid);
EMFAC®-1202; HEXACID® C-9; PELARGON®;
SCYTHE®; WEST AGRO ACID SANITIZER®
Safety Profile
Poison by intravenous
route. Moderately toxic by ingestion. A
severe skin and eye irritant. When heated to
decomposition it emits acrid smoke and
irritating fumes.
Synthesis
By oxidation of methylnonyl ketone; by oxidation of oleic acid; or from heptyl iodide via malonic ester synthesis
Potential Exposure
Pelargonic acid, a naturally occurring
fatty acid herbicide/fungicide. It is used to control the
growth of weeds and as a blossom thinner for apple and
pear trees. It is also used as a food additive; as an ingredient
in solutions used to commercially peel fruits and
vegetables; in the manufacture of lacquers, plastics and
pharmaceuticals.
Purification Methods
Esterify the acid with ethylene glycol and distil the ester. (This removes dibasic acids as undistillable residues.) The acid is regenerated by hydrolysing the ester in the usual way and is distilled in vacuo. [Beilstein 2 IV 1018.]
Incompatibilities
Heated vapors may form explosive mixture
with air. May react violently with strong oxidizers,
bromine, 90% hydrogen peroxide, phosphorus trichloride,
silver powders or dust. Incompatible with strong bases and
silver compounds; mixture with some silver compounds
may form explosive salts of silver oxalate.
Waste Disposal
Do not discharge into drains
or sewers. Dispose of waste material as hazardous waste
using a licensed disposal contractor to an approved landfill.
Contact a licensed disposal facility about surplus and
non-recyclable solutions. Burn in a chemical incinerator
equipped with an afterburner and scrubber. Extra care must
be exercised as the material in an organic solvent is highly
flammable. Consult with environmental regulatory agencies
for guidance on acceptable disposal practices. Incineration
with effluent gas scrubbing is recommended. Containers
must be disposed of properly by following package label
directions or by contacting your local or federal environmental
control agency, or by contacting your regional EPA
office.