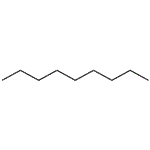
Chemical Properties
Nonane is a colorless liquid.
Chemical Properties
colourless liquid
Chemical Properties
n-Nonane, C9H20, is a colorless, highly flammable liquid.
Nonane is a constituent in the paraffin
fraction of crude oil and natural gas. It is released to the
environment via the manufacture, use, and disposal of
many products associated with the petroleum and gasoline
industries.
Physical properties
Clear, colorless, flammable liquid with a gasoline-like odor. An odor threshold concentration of
2.2 ppm
v was reported by Nagata and Takeuchi (1990).
Uses
Solvent; organic synthesis; distillation
chaser; major ingredient of such petroleum
fractions as VM&P naphtha, 140 flash, Stoddard
solvent, and gasoline
Uses
Nonane is used in organic synthesis, as a solvent, as a distillation chaser and as a fuel additive. It is also used in biodegradable detergent. It is an active ingredient of such petroleum fractions as varnish makers and painter naphtha, 140 flash, stoddard solvents and in gasoline.
Uses
Organic synthesis, biodegradable detergents,
distillation chaser.
Definition
ChEBI: A straight chain alkane composed of 9 carbon atoms.
General Description
A clear colorless liquid with a sharp odor. Flash point 86°F. Insoluble in water and less dense than water. Contact may irritate eyes and possibly injury the cornea. May irritate skin. Vapor inhalation may cause irritation. Prolonged inhalation may lead to breathing difficulty. Ingestion causes abdominal discomfort, nausea and diarrhea.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
NONANE is incompatible with oxidizing materials. N-NONANE is also incompatible with oxygen. .
Hazard
Flammable, moderate fire risk. Irritant, narcotic in high concentration. Central nervous system
impairment.
Health Hazard
Inhalation of concentrated vapor causes depression, irritation of respiratory tract, and pulmonary edema. Liquid can irritate eyes and (on prolonged contact) skin. Ingestion causes irritation of mouth and stomach. Aspiration causes severe lung irritation, rapidly developing pulmonary edema, and central nervous system excitement followed by depression.
Safety Profile
Poison by intravenous
route. Mildly toxic by inhalation. Irritating to
respiratory tract. Narcotic in high
concentrations. A very dangerous fire
hazard when exposed to heat or flame; can
react with oxidizing materials. Explosive in
the form of vapor when exposed to heat or
flame. Emitted from modern building
materials (CENEAR 69,22,91). To fight fire,
use CO2, dry chemical. When heated to
decomposition it emits acrid smoke and
irritating fumes.
Potential Exposure
Nonane is used in the synthesis of biodegradable
detergents as a distillation chaser; an ingredient
in Stoddard solvent and gasoline.
Source
Schauer et al. (1999) reported nonane in a diesel-powered medium-duty truck exhaust at
an emission rate of 160 μg/km.
Identified as one of 140 volatile constituents in used soybean oils collected from a processing
plant that fried various beef, chicken, and veal products (Takeoka et al., 1996).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rate of nonane was 3.9 mg/kg of pine burned. Emission rates of nonane were not measured during
the combustion of oak and eucalyptus.
California Phase II reformulated gasoline contained nonane at a concentration of 2.08 g/kg.
Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without catalytic
converters were 0.43 and 45.3 mg/km, respectively (Schauer et al., 2002).
Environmental Fate
Biological. Nonane may biodegrade in two ways. This first is the formation of nonyl hydroperoxide,
which decomposes to 1-nonanol followed by oxidation to nonanoic acid. The other
pathway involves dehydrogenation to 1-nonene, which may react with water giving 1-nonanol
(Dugan, 1972). Microorganisms can oxidize alkanes under aerobic conditions. The most common
degradative pathway involves the oxidation of the terminal methyl group forming 1-nonanol. The
alcohol may undergo a series of dehydrogenation steps forming nonanal then a fatty acid
(nonanoic acid). The fatty acid may then be metabolized by β-oxidation to form the mineralization
products, carbon dioxide, and water (Singer and Finnerty, 1984).
Photolytic. Atkinson reported a photooxidation rate constant of 1.02 x 10
-11 cm
3/molecule?sec
for the reaction of nonane and OH radicals in the atmosphere. Photooxidation reaction rate
constants of 1.0 x 10
-11 and 2.30 x 10
-16 cm
3/molecule?sec were reported for the reaction of nonane
with OH and NO3, respectively (Sablji? and Güsten, 1990).
Chemical/Physical. Complete combustion in air yields carbon dioxide and water.
Shipping
UN1920 Nonanes, Hazard Class: 3; Labels:
3-Flammable liquid.
Purification Methods
Fractionally distil n-nonane, then stir it with successive volumes of conc H2SO4 for 12hours each until no further coloration is observed in the acid layer. Then wash it with water, dry with MgSO4 and fractionally distil it. Alternatively, it is purified by azeotropic distillation with 2-ethoxyethanol, followed by washing out the alcohol with water, drying and distilling it. [Forziati et al. J Res Nat Bur Stand 36 129 1946, Beilstein 1 IV 447.]
Incompatibilities
Nonane forms explosive mixture with
air. Incompatible with oxidizers (chlorates, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides.
Toxics Screening Level
The initial threshold screening level (ITSL) for n-nonane is 550 μg/m3 based on an annual averaging time.
Waste Disposal
Dissolve or mix the material
with a combustible solvent and burn in a chemical
incinerator equipped with an afterburner and scrubber.
All federal, state, and local environmental regulations
must be observed.