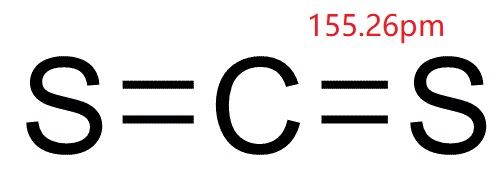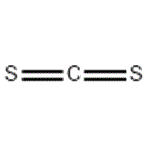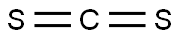
Carbon disulfide
- Product NameCarbon disulfide
- CAS75-15-0
- CBNumberCB6279761
-
MFCS2
Lewis structure
- MW76.14
- EINECS200-843-6
- MDL NumberMFCD00011321
- MOL File75-15-0.mol
- MSDS FileSDS
Chemical Properties
| Melting point | -112--111 °C (lit.) |
| Boiling point | 46 °C (lit.) |
| Density | 1.266 g/mL at 25 °C (lit.) |
| vapor density | 2.67 (vs air) |
| vapor pressure | 5.83 psi ( 20 °C) |
| refractive index | n |
| Flash point | −29 °F |
| storage temp. | 2-8°C |
| solubility | Soluble in alcohol, ether, benzene, oil, chloroform and carbon tetrachloride. |
| form | Liquid |
| Specific Gravity | 1.26 |
| color | ≤10(APHA) |
| Odor | Cabbage-like odor detectable at 0.016 to 0.42 ppm (mean = 0.2 ppm) |
| Relative polarity | 0.065 |
| explosive limit | 1-60%(V) |
| Odor Threshold | 0.21ppm |
| Evaporation Rate | 22.6 |
| Relative density, gas (air=1) | 2.63 |
Safety
| Symbol(GHS) |
  
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H225-H315-H319-H332-H361fd-H372 | |||||||||
| Precautionary statements | P201-P210-P303+P361+P353-P304+P340+P312-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | F,T | |||||||||
| Risk Statements | 11-36/38-48/23-62-63-39/23/24/25-23/24/25-48/20-40-36/37/38-19 | |||||||||
| Safety Statements | 16-33-36/37-45-7-26 | |||||||||
| RIDADR | UN 1131 3/PG 1 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 1 ppm (3 mg/m3), STEL: 10 ppm (30 mg/m3) [skin] | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | FF6650000 | |||||||||
| Autoignition Temperature | 90 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2813 10 00 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | I | |||||||||
| Hazardous Substances Data | 75-15-0(Hazardous Substances Data) | |||||||||
| Toxicity | LC50 inhal (rat) 25,000 mg/m3 (2 h) STEL (OSHA) 12 ppm (36 mg/m3)-skin PEL (OSHA) 4 ppm (12 mg/m3) TLV-TWA (ACGIH) 10 ppm (31 mg/m3)-skin | |||||||||
| IDLA | 500 ppm | |||||||||
| NFPA 704: |
|