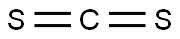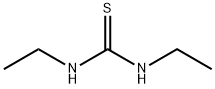
N,N'-Diethylthiourea
- Product NameN,N'-Diethylthiourea
- CAS105-55-5
- CBNumberCB2438858
- MFC5H12N2S
- MW132.23
- EINECS203-308-5
- MDL NumberMFCD00004925
- MOL File105-55-5.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 76-78 °C(lit.) |
| Boiling point | 245°C (estimate) |
| Density | 1,12 g/cm3 |
| bulk density | 735kg/m3 |
| vapor pressure | 0.001Pa at 25℃ |
| refractive index | 1.5300 (estimate) |
| Flash point | 186 °C |
| storage temp. | Store below +30°C. |
| solubility | 42g/l |
| form | solid |
| pka | 14.47±0.70(Predicted) |
| color | White to Off-White |
| PH | 7 (38g/l, H2O, 20℃) |
| Water Solubility | 38 g/L (20 ºC) |
| BRN | 773905 |
| Stability | Stable. May be air sensitive. Incompatible with strong oxidizing agents. |
| InChI | 1S/C5H12N2S/c1-3-6-5(8)7-4-2/h3-4H2,1-2H3,(H2,6,7,8) |
| InChIKey | FLVIGYVXZHLUHP-UHFFFAOYSA-N |
Safety
| Symbol(GHS) |
  
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H302+H312-H317-H318-H351-H412 | |||||||||
| Precautionary statements | P273-P280-P301+P312-P302+P352+P312-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | Xn,T | |||||||||
| Risk Statements | 22-40-45-20/21/22 | |||||||||
| Safety Statements | 45-36/37-53-24/25 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | YS9800000 | |||||||||
| TSCA | TSCA listed | |||||||||
| HS Code | 29309070 | |||||||||
| Storage Class | 11 - Combustible Solids | |||||||||
| Hazard Classifications | Acute Tox. 4 Dermal Acute Tox. 4 Oral Aquatic Chronic 3 Carc. 2 Eye Dam. 1 Skin Sens. 1B | |||||||||
| Hazardous Substances Data | 105-55-5(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|