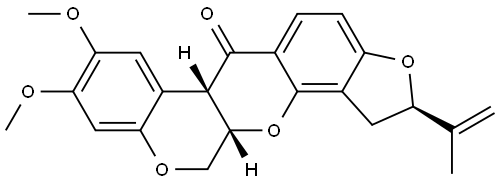
Description
The principal source of rotenone is the tuber root of
Derris elliptica; however, it is also extracted from the
roots of Derris mallaccensis, Lonchocarpus utilis, and
Lonchocarpus uruca. Rotenone is both a stomach and
contact poison for arthropods. Its fast knockdown action
is attributed to decreasing the availability of nicotinamide
adenine dinucleotide to serve as a cofactor in various
biochemical pathways including the Krebs cycle, thereby
inhibiting the mitochondrial respiratory enzymes.
Description
Rotenone is a classical inhibitor of complex I of the mitochondrial electron transport chain, inhibiting NADH/DB oxidoreductase and NADH oxidase with IC
50 values of 28.8 and 5.1 nM, respectively. In substantia nigra pars compacta neurons, it activates ATP-sensitive potassium channels and increases the production of reactive oxygen species (ROS) in the mitochondria, effects that are decreased by the antioxidant trolox . In rodents, rotenone induces dopaminergic cell death in the substantia nigra, formation of cytoplasmic inclusions similar to Lewy bodies, oxidative damage to proteins, and parkinsonian symptoms of bradykinesia and rigidity. In a rat model of Parkinson’s disease, chronic rotenone administration of 1.5 and 2.5 mg/kg per day for two months reduces tyrosine hydroxylase levels in the posterior striatum and prefrontal cortex, induces catalepsy, and decreases spontaneous locomotion and exploration in the open field test. Formulations containing rotenone have been used as insecticides and piscicides.
Chemical Properties
Rotenone is a colorless to red odorless crystalline solid; a white crystalline solid when pure; oxidation will cause yellowing to bright red coloring. Odorless.
Rotenone is related to isoflavonoid compounds derived from the roots of Derris spp., Lonchocarpus spp., and Tephrosia spp., found primarily in Southeast Asia, South America, and East Africa. The isolated compound is an odorless, colorless to red crystalline solid. It is insoluble in water and has a very low vapor pressure (U.S. EPA, 2007; HSDB, 2012a).
Uses
Rotenone is an broad spectrum insecticide that occurs naturally in seeds and stems of several plants. Rotenone was first registered in 1947 and is currently used exclusively to kill fish (U.S. EPA, 2007). In 2006, registrants voluntarily canceled all livestock, residential and home owner uses, domestic pet uses, and all other uses except for piscicide uses. Currently the main uses include fish management strategies to remove nonnative fish species from lakes, ponds, or streams and in catfish aquaculture prior to stocking ponds with with fry to remove undesirable fish species (U.S. EPA, 2006d).
Rotenone has been historically used by native people to paralyze fish for capture and consumption. Outside the United States, the compound is still used to control insects in fruit and vegetable cultivation and for control of fire ants and mosquito larvae in pond water (HSDB, 2012a).
Uses
Insecticide; lotion for chiggers;
emulsion for scabies.
Uses
Rotenone is used for the control of aphids, thrips, suckers and
other insects in fruit and vegetable cultivation. It is also used for control in
buildings, for the control of lice, ticks and warble fly on animals and as a
piscicide for the management of fish populations.
Definition
ChEBI: A member of the class of rotenones that consists of 1,2,12,12a-tetrahydrochromeno[3,4-b]furo[2,3-h]chromen-6(6aH)-one substituted at position 2 by a prop-1-en-2-yl group and at positions 8 and 9 by methoxy group
(the 2R,6aS,12aS-isomer). A non-systemic insecticide, it is the principal insecticidal constituent of derris (the dried rhizome and root of Derris elliptica).
General Description
Colorless to brownish crystals or a white to brownish-white crystalline powder. Has neither odor nor taste.
Air & Water Reactions
ROTENONE decomposes upon exposure to light or air. Insoluble in water.
Reactivity Profile
ROTENONE is readily oxidized in the presence of alkalis. ROTENONE is incompatible with oxidizers. .
Hazard
Toxic by ingestion, overexposure can be
fatal, irritant to skin, eyes and upper respiratory
tract. Central nervous system impairment. Ques-
tionable carcinogen.
Health Hazard
Rotenone is an irritant and affects
the nervous system, causing convulsions.
Fire Hazard
Flash point data for ROTENONE are not available; however, ROTENONE is probably combustible.
Agricultural Uses
Insecticide, Acaracide, Veterinary medicine: The use of rotenone as a pesticide to kill invasive fish species is currently the only allowable use of this pesticide. Rotenone is a selective, nonspecific botanical insecticide with some acaricidal properties, and has been used in agriculture to control insects on vine fruit, flowers and vegetables. Registered for use in the U.S.A U.S. EPA restricted Use Pesticide (RUP) due to acute inhalation, acute oral, and aquatic toxicity. Agricultural and residential uses and all food uses were voluntarily cancelled in 2006[83]. In 2006, registrants requested voluntarily cancellation of all livestock, residential and home owner uses, domestic pet uses, and all other uses except for pesticide uses. In 2011 the use of this pesticide chemical was linked to Parkinson’s disease. Not listed for use in EU countries.
Trade name
ACME® Rotenone; AROL GORDON DUST®; BARBASCO®; BONIDE CUKE AND MELON DUST®; CENOL GARDEN DUST®; CHEM FISH®; CHEM-MITE®; CUBE®; CUBE EXTRACT®; CUBEPULVER®; CUBEROL®; CUBE ROOT®; CUBOR®; CUREX FLEA DUSTER®; DACTINOL®; DERIL®; DERRIN®; DERRIS®; DRI-KIL®; ENT-133®; EXTRAX®; FISH-TOX®; GREEN CROSS WARBLE POWDER®; HAIARI®; LIQUID DERRIS®; MEXIDE®; NICOULINE®; NOXFIRE®; NOXFISH®; PARADERIL®; POWDER AND ROOT®; PRENTOX®; PRO-NOX FISH®; RO-KO®; RONONE®; ROTACIDE®; ROTEFIVE®; ROTEFOUR®; ROTESSENOL®; SINID®; TOX-R®; TUBATOXIN®
Biological Activity
Mitochondrial electron transport chain inhibitor (IC 50 = 1.7 - 2.2 μ M at complex I). Inhibits NADH oxidation by cardiac sarcoplasmic reticulum (IC 50 = 3.4 nM). Commonly used pesticide and induces Parkinsonism in animal models. Cell-permeable and brain penetrant.
Biochem/physiol Actions
Rotenone is an inhibitor of mitochondrial electron transport at nicotinamide adenine dinucleotide (NADH):ubiquinone oxidoreductase. It is readily absorbed through the exoskeletons of arthropods, but poorly absorbed cutaneously or from the gastrointestinal tract of mammals. Rotenone acts as a neurotoxic agent which can produce Parkinson-like condition to serve as an animal model for the study of etiology and interventions.
Potential Exposure
A potential danger to those involved
in extraction from derris root, formulation or application of
this insecticide. Rotenone is used as a pharmaceutical and
veterinary drug.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Speed in removing material from skinis of extreme importance. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions,including resuscitation mask) if breathing has stopped andCPR if heart action has stopped. Transfer promptly to amedical facility. When this chemical has been swallowed,get medical attention. Give large quantities of water andinduce vomiting. Do not make an unconscious personvomit. Medical observation is recommended for 24-48 hafter breathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
Carcinogenicity
In human lymphocyte culture assays
rotenone did not increase the frequency of
chromosomal aberrations or sister chromatid
exchanges but did cause an increase in the frequency
of binucleated micronuclei and a delay
in cell cycle.
Environmental Fate
Rotenone released to the atmosphere will exist as particulates due to the extremely low vapor pressure. Particulate-phase rotenone will be removed from the atmosphere by wet and dry deposition and may be degraded by direct photolysis. It is mobile to moderately mobile in soil and sediment and volatilization from soil surfaces is not expected to occur to any extent. If released to water, rotenone generally degrades quickly through abiotic (hydrolytic and photolytic) mechanisms, with half-lives of a few days to several weeks or longer depending on water temperature (U.S. EPA, 2007; HSDB, 2012a).
Rotenone has a relatively low potential for bioconcentration in aquatic organisms (Bioconcentration Factor (BCF) < 30X) (U.S. EPA, 2007).
Metabolic pathway
By hepatic microsomal incubations from rainbow trout
with 14C-rotenone, three major and several minor
metabolites of rotenone are observed, the major ones
being identified as rotenolone and two epimeric forms
of 6' ,7' -dihydroxyrotenone.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required. UN2588 Pesticides, solid, toxic, Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required.
Degradation
Rotenone is racemised in base to less insecticidal compounds and it is
decomposed on exposure to light and air (PM). Photochemical degradation
in methanol of seven rotenoids isolated from Tephrosa villosa
(Krupadanam et al., 1978) resulted generally in much decomposition but
rotenone was relatively stable under the conditions used. When the compound
was used as an insecticidal spray it was converted by light and air
into dehydro-rotenone (2) and rotenonone (3). These structures are shown
in Scheme 1.
Toxicity evaluation
Rotenone inhibits the electron transport chain by blocking
transport between the flavoprotein and the ubiquinone. The
oxidation of pyruvate in rat mitochondria is virtually
completely blocked by rotenone in vitro (<1 mmol l
-1 concentration). Cell death occurs by apoptosis due to excess
generation of free radicals. In addition, rotenone causes a definite
anesthetic effect when it comes in contact with nerve
axons. Death appears to occur due to depression of the respiratory
center.
Rotenone is toxic to insects, humans, animals, and fish.
Rotenone exerts selective toxicity, as it is highly toxic to fish
because of its rapid absorption from the GI tract in comparison
to mammalian species in which it is poorly absorbed. The
selective toxicity of rotenone in insects and fish versus
mammals can also be explained based on the metabolism of
this compound. Rotenone converts to highly toxic metabolites
in large quantities in insects and fish, while it converts to
nontoxic metabolites in mammals.
Incompatibilities
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explo-
sions. Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides, and alkalies.
Waste Disposal
Rotenone is decomposed by
light and alkali to less insecticidal products. It is readily
detoxified by the action of light and air. It is also detoxified
by heating; 2 hours @ 100 ? C results in 76% decomposition.
Oxidation products are probably nontoxic. Incineration has
been recommended as a disposal procedure. Burial with
lime would also present minimal danger to the environ-
ment . In accordance with 40CFR165, follow recommen-
dations for the disposal of pesticides and pesticide
containers. Must be disposed properly by following pack-
age label directions or by contacting your local or federal
environmental control agency, or by contacting your
regional EPA office.
References
[1]. chen y, mcmillan-ward e, kong j, et al. mitochondrial electron-transport-chain inhibitors of complexes i and ii induce autophagic cell death mediated by reactive oxygen species. j cell sci, 2007, 120(pt 23): 4155-4166.
[2]. newhouse k, hsuan sl, chang sh, et al. rotenone-induced apoptosis is mediated by p38 and jnk map kinases in human dopaminergic sh-sy5y cells. toxicol sci, 2004, 79(1): 137-146.
[3]. borland mk, trimmer pa, rubinstein jd, et al. chronic, low-dose rotenone reproduces lewy neurites found in early stages of parkinson's disease, reduces mitochondrial movement and slowly kills differentiated sh-sy5y neural cells. mol neurodegener, 2008, 3: 21.