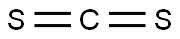Carbon disulfide: CS2 Lewis Structure;molecular geometry;hybridization
CS2 Lewis Structure
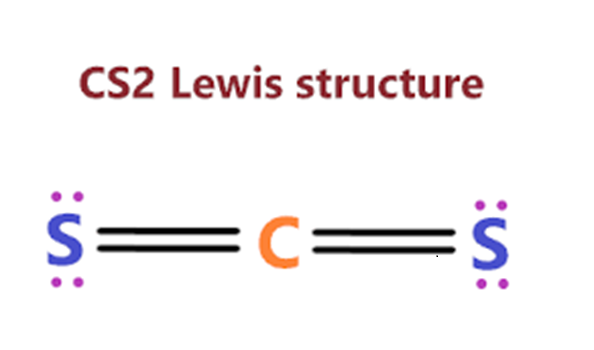
The CS2 Lewis structure consists of a central carbon atom (C) and two external sulphur atoms (S) bonded at 180°. The carbon (C) and sulphur (S) atoms are each connected by a double bond. Each sulphur atom (S) has two lone pairs of electrons. The CS2 Lewis structure is shown below:
Steps for drawing the CS2 Lewis structure
Step 1 Calculate the number of valence electrons for C and S
Carbon and sulphur are elements of groups 14 and 16 of the periodic table. Therefore, there are 4 and 6 valence electrons in the carbon and sulphur atoms respectively, so the total number of valence electrons in the CS2 molecule = valence electrons from 1 carbon atom + valence electrons from 2 sulphur atoms = 4 + 6(2) = 16.
Step 2 Identify the central atom
The central atom must be highly or minimally electronegative. For the CS2 molecule, the carbon atom (C) is less electronegative than the sulphur atom, so carbon is the central atom and sulphur is the outer atom.
Step 3 Labelling the electron lone pairs between atoms
Total number of valence electron pairs = σ-bonds + π-bonds + lone pairs of electrons in the valence layer, i.e., the total number of valence electron pairs divided by 2. For the CS2 molecule, the total number of pairs of electrons is eight. The carbon atom is connected to each of the 2 sulphur atoms by a σ-bond (one σ-bond equals one pair of electrons), and since we are left with 12 valence electrons, we must first place these electrons around the outer atom (sulphur) in order to complete its octet rule. Thus, there are 3 lone pairs of electrons on each sulfur atom (2).
Step 4 Stability of structure and minimize charges on atoms by converting lone pairs to bonds
When there are positive and negative charges on lot of atoms or higher charges (like +2, +3, -2, -3) on atoms in an ion or molecule, that structure is not stable. Therefore, We should try to reduce charges on atoms if it is a possible.
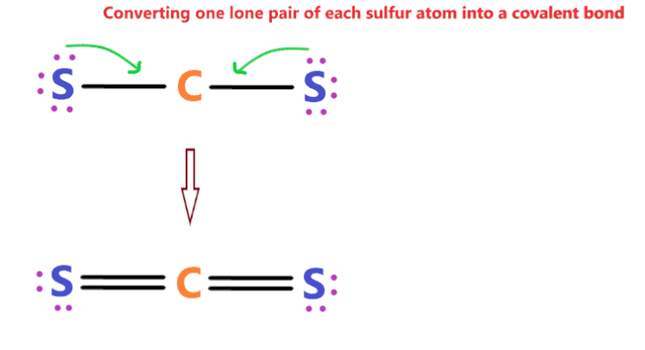
In order to check the stability of the central carbon (C) atom, we have to check whether it is forming an octet or not, moreover, we do not have extra valence electrons for completing the octet of carbon. Therefore, to overcome this problem, we will take the help of sulphur lone pair of electrons. In order to stabilise this carbon atom, the electron pair has to be moved away from the outer sulphur atom so that the carbon atom can have eight electrons (i.e. octet). After the move, each of the two sulphur atoms forms a double bond with the carbon atom, retaining two lone pair electrons on each sulphur atom. Both atoms (carbon and sulphur) have completed their octet rules and both have reached a stable structure. See the diagram below.
Molecular geometry
The molecular geometry of CS2 is linear. This is because the carbon (C) centre atom has no lone pair of electrons and is attached to two sulphur (S) atoms. Therefore, there are two regions of electron density around the carbon centre atom, which, according to the VSEPR theory, will give a linear molecular geometry.
Hybridization
According to the VSEPR theory, the space number is used to determine the hybridisation of the atom. According to the CS2 Lewis structure, carbon is the central atom with zero lone pair of electrons and two sulphur atoms attached to it. Therefore, the space number = 0 + 2, so two space numbers means that carbon has Sp hybridisation in the CS2 molecule.
You may like
Related articles And Qustion
See also
Lastest Price from Carbon disulfide manufacturers

US $15.00-10.00/KG2021-07-13
- CAS:
- 75-15-0
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton

US $15.00-10.00/KG2021-07-10
- CAS:
- 75-15-0
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton