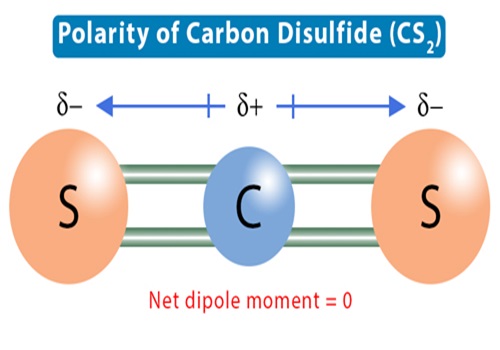
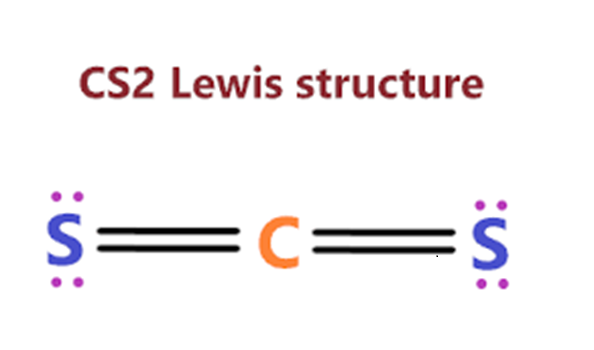
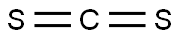Uses of carbon disulfide
For many years, carbon disulfide was manufactured by the reaction of charcoal with sulfur vapor at temperatures of 750– 1000°C, but by the mid-twentieth century, especially in the United States, the process was superseded by the reaction of natural gas (principally methane) with sulfur.

Uses
The principal industrial uses of carbon disulfide include the
manufacturing of cellophane film, viscose rayon, xanthogenates,
carbon tetrachloride, and electronic vacuum tubes.
Carbon disulfide is used for fumigation in airtight flat
storages, airtight storage warehouses, bins, grain elevators,
shipholds, barges, and cereal mills. It is also used as an insecticide
for the fumigation of grains, in fresh fruit conservation,
nursery stock, and as a soil disinfectant against insects and
nematodes. Carbon disulfide is a solvent for selenium, phosphorus,
sulfur, iodine, bromine, fats, resins, and rubber. It is
also used in the purification of single-walled carbon nanotubes.
Environmental Fate
Carbon disulfide is a clear, colorless or faintly yellow, mobile liquid at room temperature, and has an ‘ether-like’ odor. It is highly flammable and volatile. It has a solubility of 2160 mg l-1 in water at 25°C, and is very slightly soluble in water, as well as in alcohol, benzene, ether, chloroform, carbon tetrachloride, and oils. If released to air, an estimated vapor pressure of 359 mmHg at 25°C indicates that carbon disulfide will exist solely as a vapor in the ambient atmosphere and may potentially volatilize from dry soil surfaces given its vapor pressure. Based on the estimated Henry’s law constant of 1.44×102 atm-m3 mol-1 at 24°C for carbon disulfide, volatilization is expected to occur from moist soil surfaces and rapidly from water surfaces. Other physical properties include an octanol/water partition coefficient as log Pow of 1.84, a boiling point of 46°C, and a melting point of 111°C.
Mechanism of Toxicity
Carbon disulfide reacts with a variety of important nucleophilic compounds in the body (e.g., pyridoxamine, cerebral monoamine oxidases, dopamine carboxylases, amino acids, biogenic amines, and sugars). Acute CNS toxicity and peripheral neurotoxicity caused by carbon disulfide may result from formation of dithiocarbamates. Carbon disulfide may react with macromolecules of enzymes, structural proteins, polypeptides, and nucleic acids. Chelation of zinc- or coppercontaining enzymes by carbon disulfide metabolites has been proposed as one of the mechanisms by which carbon disulfide induces neurotoxicity.
Carbon disulfide alters the metabolism of vitamin B6 and nicotinic acid and causes vitamin B deficiency. It is an inhibitor of brain monoamine oxidase that leads to impairment of catecholamine metabolism. Carbon disulfide affects liver enzymes, particularly those related to lipid metabolism, which finally results in higher serum cholesterol. Carbon disulfide also interacts with microsomal drug metabolism through deactivation of cytochrome P450.
You may like
Related articles And Qustion
Lastest Price from Carbon disulfide manufacturers

US $15.00-10.00/KG2021-07-13
- CAS:
- 75-15-0
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton

US $15.00-10.00/KG2021-07-10
- CAS:
- 75-15-0
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton