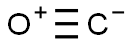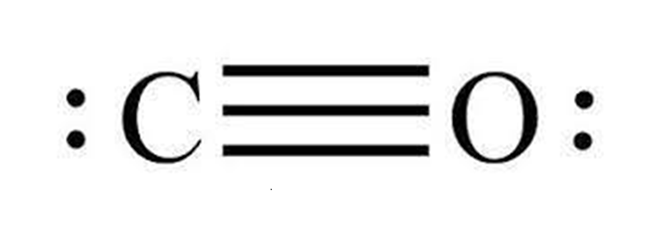Mechanism of Carbon monoxide
Carbon monoxide (CO) is historically known as a deadly gas to humans. Although produced in small amounts in the human body, toxicity occurs predominately after inhalation of preformed CO. Exposure to CO can occur in occupational settings, in tobacco smoke, and in home settings whenever there is incomplete combustion of carbon-containing materials in an unventilated setting.
Uses
CO is used in industries as a feedstock for the production of methanol, acrylates, phosgene, and ethylene. It is also used in metallurgy applications and in industrial fuels. It is also recently being studied in preclinical stages for medicinal use. A major source of CO is the incomplete combustion of carboncontaining materials.
Environmental Fate
Exposure to this colorless, odorless gas occurs primarily though
inhalation. CO exposure associated with the paint stripper
methylene chloride is unique in that methylene chloride is biologically
metabolized toCOin vivo. Dermal, oral, and inhalation
exposure to methylene chloride can cause CO poisoning.
Aside from tobacco smoke, the most important sources of
CO exposure for most individuals are the emissions created by
internal combustion engines of vehicles and in household and
occupational locations where combustion occurs. Specific sources
of exposure include the burning of wood, charcoal, natural
gas, or propane for heating and cooking, and propane-powered
indoor equipment such as forklifts and ice rink resurfacers.
Average levels of CO in homes without gas stoves vary from
0.5 to 5 ppm. Levels near properly adjusted gas stoves are often
5–15 ppm, and those near poorly adjusted stoves may be
30 ppm or higher. CO exposures occur in a variety of occupational
settings. The number of persons occupationally exposed
to CO in the working environment is greater than for any other
physical or chemical agent. The smoke of a cigarette contains
approximately 14 mg of CO. The smoke of cigars ranges from
approximately 38 mg for little cigars to almost 100 mg for large
and premium cigars. CO in secondhand tobacco smoke has led
to levels of CO as high as 50 ppm.
Mechanism of Toxicity
CO has varied effects on multiple enzymatic reactions and
processes. Most easily seen and measured via co-oximetry is its
high affinity and binding to Hb. This results in an overall lack
of oxygen carrying capacity along with a shift of the oxygen
dissociation curve to the left so that even available oxyhemoglobin
is less able to offload oxygen to tissue sites. This,coupled with CO’s ability to bind to and arrest cellular
metabolism, results in global hypoxemia. The overall lack of
tissue perfusion and energy production results in metabolic
lactic acidosis.
CO also has the ability to bind to other globins, most
importantly myoglobin. Significant myoglobin binding
results in lack of tissue oxygenation to heart and myocardial
damage.
The final high-risk organ system affected after CO exposure
is the central nervous system. CO has the ability to cause
delayed neuropsychiatric sequelae in addition to the acute
effects seen as a result of hypoxemia. This is thought to be due
to delayed lipid peroxidation achieved through the displacement
of nitric oxide. A reperfusion-like injury occurs in these
cases.