The structure and mechanism of action of 5-Fluorouracil
Description
5-Fluorouracil, also known as 5-FU, is the third most commonly used chemotherapeutic agent in the treatment of solid malignancies across the world, including head, neck, and gastrointestinal tumors. Fluoropyrimidines also possess radiosensitizing properties and are often used with external beam radiotherapyp. It is in the chemotherapy class of drugs. Since 1957, it has played an essential role in the treatment of colon cancer and is used for patients with breast and other cancers, like those of the head and neck.
structure
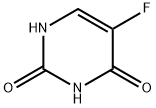
5-FU is a heterocyclic aromatic organic compound with a structure similar to that of the pyrimidine molecules of DNA and RNA; it is an analog of uracil with a fluorine atom at the C-5 position in place of hydrogen. Only one crystal structure is reported in the literature for pure 5-FU, in which the compound crystallizes with four molecules in the asymmetric unit, and the molecule adopts a hydrogen-bonded sheet structure[1]. Due to its structure, 5-FU interferes with nucleoside metabolism and can be incorporated into RNA and DNA, leading to cytotoxicity and cell death.
Mechanism of action
Antimetabolite drugs work by inhibiting essential biosynthetic processes or being incorporated into macromolecules, such as DNA and RNA, and inhibiting their normal function. The fluoropyrimidine 5-fluorouracil (5-FU) does both.
5-FU is an analog of uracil with a fluorine atom at the C-5 position instead of hydrogen. It rapidly enters the cell using the exact facilitated transport mechanism as uracil. 5-FU is converted intracellularly to several active metabolites: fluorodeoxyuridine monophosphate (FdUMP), fluorodeoxyuridine triphosphate (FdUTP), and fluorouridine triphosphate (FUTP)— these active metabolites disrupt RNA synthesis and the action of TS. The rate-limiting enzyme in 5-FU catabolism is dihydropyrimidine dehydrogenase (DPD), which converts 5-FU to dihydrofluorouracil (DHFU). More than 80% of administered 5-FU is normally catabolized primarily in the liver, where DPD is abundantly expressed[3].
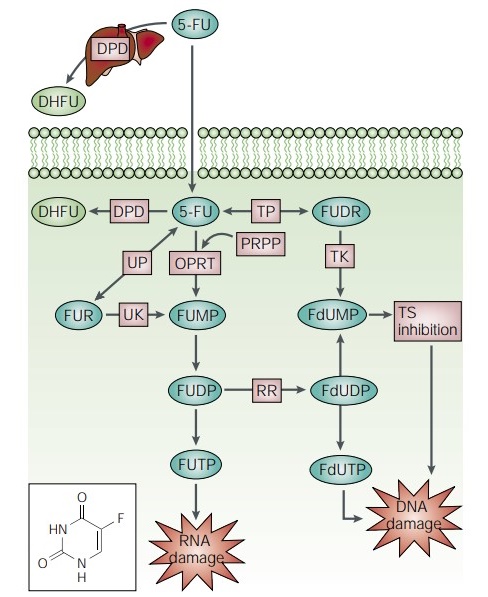
The 5-FU metabolite FdUMP binds to the nucleotide-binding site of TS, forming a stable TERNARY COMPLEX with the enzyme and CH2 THF, thereby blocking the binding of the normal substrate dUMP and inhibiting dTMP synthesis. The 5-FU metabolite FUTP is extensively incorporated into RNA, disrupting normal RNA processing and function. 5-FU misincorporation can result in toxicity to RNA at several levels. It not only inhibits pre-rRNA processing into mature rRNA but also disrupts post-transcriptional modification of tRNAs and the assembly and activity of snRNA/protein complexes, thereby inhibiting the splicing of pre-mRNA.
Side effects
Nevertheless, as with other chemotherapeutic agents, the potential benefits of fluoropyrimidines have to be weighed against their risks and drug-related toxicities. 5-FU is the second most common drug associated with cardiotoxicity after anthracyclines. The most common manifestation of cardiotoxicity associated with fluoropyrimidines is chest pain, presenting as atypical chest pain, angina on exertion or rest, and acute coronary syndromes, including myocardial infarction. Other less common manifestations of cardiotoxicity include atrial fibrillation and other arrhythmias, myocarditis and pericarditis, heart failure, and even death. Fluoropyrimidine-related cardiotoxicity, however, remains a poorly defined entity.
References
[1] Ning Zhang. “5-Fluorouracil: mechanisms of resistance and reversal strategies.” Molecules 13 8 (2008): 1551–69.
[2] Daniel B Longley, Patrick G Johnston, D Paul Harkin. “5-fluorouracil: mechanisms of action and clinical strategies.” Nature Reviews Cancer 3 5 (2003): 330–8.
[3] W.J. Gradishar, E.E. Vokes. “Review: 5-Fluorouracil cardiotoxicity: A critical review.” Annals of Oncology 1 6 (1990): Pages 409-414.
You may like
Related articles And Qustion
See also
Lastest Price from 5-Fluorouracil manufacturers

US $0.00/kg2025-08-26
- CAS:
- 51-21-8
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $5.00-0.50/KG2025-06-07
- CAS:
- 51-21-8
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg