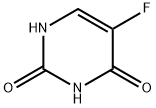
What cancers does 5-fluorouracil treat for?
5-fluorouracil (5-FU) is a drug given as an injection to treat cancers of the breast, colon, rectum, stomach, and pancreas. Fluorouracil cream and topical solution treat actinic or solar keratoses (scaly or crusted lesions [skin areas] caused by years of too much exposure to sunlight). Fluorouracil cream and topical solution are also used to treat a type of skin cancer called superficial basal cell carcinoma if usual types of treatment cannot be used. It is used under the brand names Carac, Tolak, Efudex, and Fluoroplex to treat actinic keratosis. It is also used under the brand name Efudex to treat basal cell skin cancer. 5-fluorouracil is also being studied to treat other conditions and types of cancer. It stops cells from making DNA and may kill cancer cells. 5-fluorouracil is a type of antimetabolite. Also called 5-FU and fluorouracil[1].
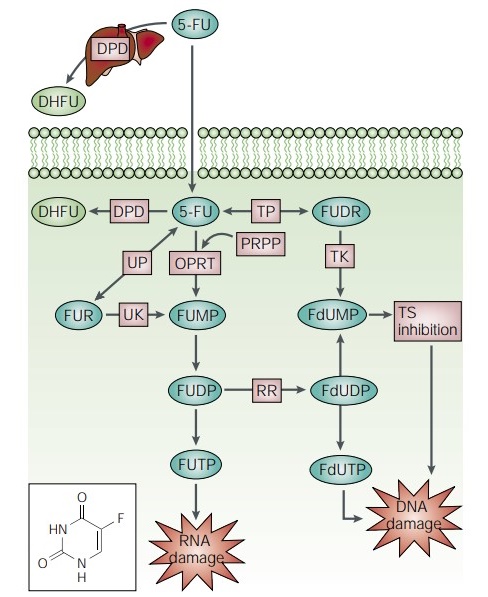
In mammalian cells, 5-FU is converted to fluorodeoxyuridine monophosphate (FdUMP), which forms a stable complex with thymidylate synthase (TS) and thus inhibits deoxythymidine mono-phosphate (dTMP) production. dTMP is essential for DNA replication and repair, and its depletion causes cytotoxicity. Dihydropyrimidine dehydrogenase (DPD)-mediated conversion of 5-FU to dihydrofluorouracil (DHFU) is the rate-limiting step of 5-FU catabolism in normal and tumor cells. Up to 80% of administered 5-FU is broken down by DPD in the liver.
Research has indicated that 5-FU exerts its anticancer effects mainly through inhibition of TS, for which the pathways have not been fully interpreted. Santi has pointed out that the formation of the ternary TS–FdUMP–CH2THF complex is time-dependent, and the reaction stops as the fluorine substituent fails to dissociate from the pyrimidine ring, resulting in a slowly reversible inactivation of the enzyme. Reduction of dTMP leads to downstream depletion of deoxythymidine triphosphate (dTTP), which induces perturbations in the levels of the other deoxynucleotides (dATP, dGTP and dCTP). Finally, the imbalances (the ATP/dTTP ratio specifically) are thought to severely disrupt DNA synthesis and repair, resulting in lethal DNA damage. Accumulation of dUMP, which might subsequently lead to increased levels of deoxyuridine triphosphate (dUTP), can be misincorporated into DNA, and FdUTP, the metabolic product of 5-FU, has the same action. Furthermore, repairing enzyme uracil-DNA-glycosy-lase (UDG) is suggested to be useless in the presence of high (F) dUTP/dTTP ratios and only results in further false DNA repair[2].
5-FU is a pyrimidine analogue that can be misincorporated into RNA and DNA in place of uracil or thymine. The interference with the normal biosynthesis or function of nucleic acids is another possible mechanism of action for 5-FU. 5-FU can be misincorporated into the DNA of drug-treated cells, and accumulation of 5-FU in the genome, rather than uracil excision, is correlated with 5-FU cytotoxicity in mammalian cells. It can also be misincorporated into RNA, and evidence suggests that RNA-based effects play a significant role in its cytotoxicity. Experiments in yeast show that defects in the nuclear RNA exosome subunit Rrp6p could cause hypersensitivity to 5-FU. Genetic analyses suggest that while a DNA repair mutation, apn1-Δ causes sensitivity to 5-FU-induced DNA damage, and an rrp6-Δ mutation causes hypersensitivity, due to the RNA-based effects of 5-FU.
References:
[1] DANIEL B. LONGLEY P G J D Paul Harkin. 5-Fluorouracil: mechanisms of action and clinical strategies[J]. Nature Reviews Cancer, 2003, 3 5. DOI:10.1038/nrc1074.You may like
Related articles And Qustion
See also
Lastest Price from 5-Fluorouracil manufacturers

US $0.00/kg2025-08-26
- CAS:
- 51-21-8
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $5.00-0.50/KG2025-06-07
- CAS:
- 51-21-8
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg