Isophthalic acid: Chemical property and Uses
Introduction
Isophthalic acid (IPA) is a non-toxic organic compound with C6H4(CO2H)2 formula. This colourless solid is an isomer of phthalic acid and terephthalic acid. These aromatic dicarboxylic acids are precursors (in the form of acylchlorides) to commercially important polymers. The high-performance polymer polybenzimidazole is produced from isophthalic acid. It is one of the commercially main aromatic dicarboxylic acids with superior thermal, chemical, and radiation resistance and different industrial applications.
Currently, IPA has been commercially generated by liquid-phase oxidation in the presence of a suitable catalyst by utilizing air as an oxidant and acetic acid (AcOH) as a solvent. In this manufacturing technology, m-toluic acid is the main impurity.
Chemical property
IPA is a dibasic acid (with two displaceable hydrogen atoms) and has two dissociation constants (pKa1 = 3.70; pKa2 = 4.60 at 25 ℃). IPA is closely related in structure to terephthalic acid (TPA; CASRN=100-21-0). IPA and TPA are isomers, differing only with respect to the positioning of their carboxylic acid groups on the benzene ring (1,3- and 1,4-, respectively). The physical-chemical properties, metabolism pathways, and toxicological properties of IPA and TPA are very similar.
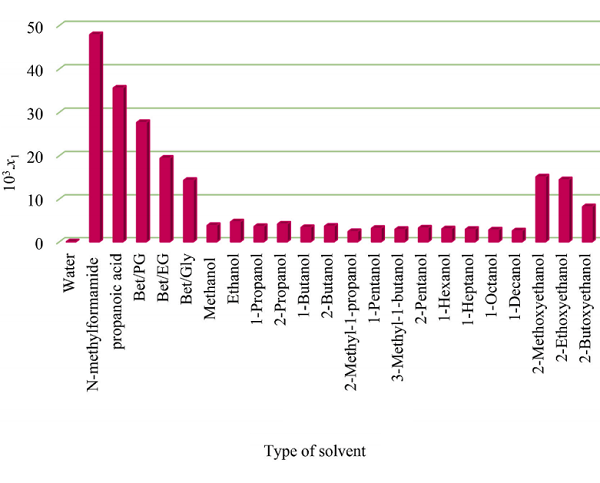
The solubilities of IPA in various organic solvents (10 3 . x1, T) follow the order: N-methyl formamide (47.9) > propanoic acid (35.6) > Bet/PG DES (27.664) > Bet/EG DES (19.431) > Bet/Gly DES (14.338) > 2- methoxy ethanol (15.130) > 2-ethoxyethanol (14.470) > 2-butoxyethanol (8.213) > ethanol (4.671) > 2-propanol (4.163) > methanol (3.835) > 2-butanol (3.656) > 1-propanol (3.598) > 1-butanol (3.368) > 2-pentanol (3.240) > 1-pentanol (3.165) > 1-hexanol (3.040) >1-heptanol (2.935) > 3-methyl-1-butanol (2.920) > 1-octanol (2.791) > 1-decanol (2.586) > 2- methyl-1-propanol (2.390)[1]. This order presents a higher solubilization power of DESs than the common organic solvents except for N-methyl formamide and propanoic acid.
Uses
Isophthalic acid is an aromatic dicarboxylic acid industrially produced by the oxidation of m-xylene. Isophthalic acid is used as a raw material in the production of polyesters. About 35% of the isophthalic acid is used to prepare unsaturated polyester resins, about 30% is used to produce alkyd coatings, and over 20% is used in other types of polymers, primarily as a minor comonomer with terephthalic acid in saturated polyesters. Isophthalic acid is also used in formulations for adhesives, inks, wire enamels, and dental materials. In addition, it is used to produce derivative chemicals such as the diesters.
Commercially, it is used primarily as a component of PET (polyethene terephthalate) copolymer, used in bottle resins and, to a much lesser extent, for fibres. Isophthalic acid reduces the crystallinity of PET, which serves to improve clarity and increase the productivity of bottle-making. Isophthalic acid’s second major use is as a component of high-quality alkyds and polyester resins for industrial coatings and unsaturated polyesters for fiberglass-reinforced plastics applications.
References:
[1] PARISA JAFARI . Determination of the solubility profile of isophthalic acid in some aqueous solutions of betaine-based deep eutectic solvents: Study the extent of H-bonding interactions between starting materials of deep eutectic solvents in aqueous medium[J]. Sustainable Chemistry and Pharmacy, 2023, 35. DOI:10.1016/j.scp.2023.101208.You may like
Related articles And Qustion
Lastest Price from Isophthalic acid manufacturers
US $0.00-0.00/KG2025-11-28
- CAS:
- 121-91-5
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $10.00/kg2025-04-21
- CAS:
- 121-91-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt