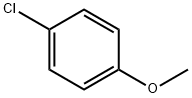
Chemical Properties
colourless liquid
Uses
4-Chloroanisole was found to react with Cu (II)-smectite forming a blue clay-organic complex. 4-Chloroanisole was chosen as the main test substrate for the optimisation studies as it is electronically deactivated and thus resistant to oxidative addition and consequently very reluctant to enter a catalytic manifold.
Synthesis Reference(s)
Chemistry Letters, 16, p. 1901, 1987
The Journal of Organic Chemistry, 35, p. 528, 1970
DOI: 10.1021/jo00827a060
General Description
4-Chloroanisole is an electron-rich chloroarene. It undergoes Ullmann-type homocoupling catalyzed by
in situ generated Pd colloids. Reaction of 4-chloroanisole with Cu(II)-smectite in
n-hexane, carbon tetrachloride or dichloromethane has been studied. The nucleophilic photosubstitution reactions, photocyanation and photohydrolysis of 4-chloroanisole has been studied by time resolved spectroscopy.
Purification Methods
Wash the anisole with 10% (by volume) aqueous H2SO4 (three times), 10% aqueous KOH (three times), and then with water until neutral. Dry it (MgSO4), and fractionally distil it from CaH2 through a glass helices-packed column under reduced pressure. [Beilstein 16 IV 822.]