Description
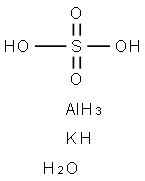
Potassium alum is aluminum potassium sulfate, also known as potash alum or tawas. Its chemical formula is K2SO4.Al2(SO4)3.24H2O. It is a semi-transparent, white-colored stone. This is the type of alum that humans find in the grocery store for pickling and in baking powder. It is also used in leather tanning, as a flocculant in water purification, as an ingredient in aftershave, and as a treatment for fireproof textiles.

Uses
Reducing perspiration is the primary use for potassium alum, as this mineral is capable of regulating the amount of sweat that is released during the day. It also leaves a thin, transparent layer on the skin that combats the growth of bacteria present in the most superficial layer of the skin and reduces the smell of sweat. Like coarse salt, potassium alum can be used to exfoliate the skin when showering, removing dead cells and stimulating circulation and collagen fiber production. This can help keep the skin healthy and reduce the appearance of red stretch marks. It also has excellent antibacterial and healing properties that help eliminate the bacteria that cause canker sores. This mineral can also speed up the healing of these lesions. Due to its antiseptic properties, potassium alum can effectively eliminate the bacteria in the first layer of the skin, promoting deep cleansing. It also helps to improve the skin's texture, closing the pores and making it more difficult for new pimples to appear. This compound can be used to stop the bleeding of small wounds and facilitate healing. It is often used by manicurists when caring for nails. This mineral helps contract the blood vessels to stop bleeding and promote healing from small lacerations.
Production Methods
Potassium Alum is usually extracted from a mineral called alunite. However, potassium alum is also industrially produced today. One of the most common preparation methods includes adding potassium sulfate in an aluminum sulfate solution in concentrated form. If the sulfate contains a higher amount of iron instead of potassium sulfate, potassium chloride can be used. The preparation method comprises mixing aluminum sulfate, potassium sulfate, and water at a temperature of 80-100 DEG C so that the materials undergo a reaction, carrying out evaporative concentration on the reaction product, adding a seed into the product, and carrying out cooling crystallization to obtain potassium alum crystals.
Safety
Potassium alum is often included in preparations for mouthwashes or gargles and dermatological preparations. Large doses of potassium alum act as an irritant and may be corrosive; gum necrosis and gastrointestinal hemorrhage have occurred. Acute encephalopathy has been reported following bladder irrigation with alum solutions in the treatment of bladder hemorrhage. Anecdotal evidence suggests that this practice should be avoided for patients with renal insufficiency.