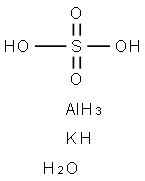
Synthesis of Aluminium potassium sulfate dodecahydrate (Alum)
Synthesis of a Hydrate
Approximately 1g of aluminum foil was weighed to the nearest centigram, torn into small pieces, and placed into a 250mL beaker. 25mL of 3M potassium hydroxide solution was added slowly and was allowed to react until the foil was dissolved. Undissolved solids were removed and discarded through vacuum filtration. The solution was allowed to cool then acidified slowly with constant stirring using 45mL of 3M sulfuric acid. The solution was then boiled until the water evaporated to give a volume of about 50mL of solution. The solution was cooled overnight. After collecting the aluminium potassium sulfate dodecahydrate crystals by vacuum filtration, they were washed with 50mL of a 50% by volume water and ethanol mixture. The crystals were then allowed to dry at room temperature, and then massed. The theoretical yield of aluminium potassium sulfate dodecahydrate (alum) was then calculated, assuming that aluminum was the limiting reactant and that the foil was 100% aluminum, and was then used to calculate the percent yield.

Analysis of a Hydrate
Part 1:
Approximately 0.5g of dry aluminium potassium sulfate dodecahydrate (alum) were pulverized with a mortar and pestle, and then packed into a capillary tube to a depth of about 1 cm. A rubber band was used to fasten the capillary tube to a thermometer with the alum level with the bulb of the thermometer. A universal clamp and cork stopper were used to fasten the thermometer to a ring stand. The bottom of the capillary tube and thermometer were immersed in a Thide-Dennis tube, which was filled with water to about 1 inch past the second arm. The sample was heated rapidly in the beginning, but slowly as it approached the melting point in order to get an accurate reading. The temperature at which the alum melts was recorded, and was compared to the published value.
Part 2:
After finding the mass of an evaporating dish and watch glass, approximately 2g of aluminium potassium sulfate dodecahydrate (alum) crystals were added to the evaporating dish. The evaporating dish, alum, and watch glass were then placed on wire gauze held in a ring on a ring stand and heated very gently over a low blue flame. After the vapor had appeared to be driven off, the alum was heated more strongly for about five minutes. After cooling, the mass of the anhydrous alum and the water driven off were calculated and compared to the accepted value of alum.
Part 3:
After weighing about 1g of alum into a 250mL beaker, it was dissolved in about 50 mL of distilled water. After calculating the volume of 0.2M barium nitrate that would be needed to totally precipitate all of the sulfate ions present in the solution, twice that amount was added slowly while stirring. The beaker was then covered with a watch glass and heated nearly to boiling, where it was kept for about 15 minutes. The precipitate was then filtered using vacuum filtration and, along with the beaker, was washed several times with small quantities of distilled water. The filter and precipitate was then transferred to an evaporating dish and allowed to dry in an oven.
You may like
See also
Lastest Price from Aluminium potassium sulfate dodecahydrate manufacturers

US $6.00/kg2025-04-21
- CAS:
- 7784-24-9
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000

US $980.00-750.00/ton2025-04-21
- CAS:
- 7784-24-9
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 5000