Chemical Properties
The PhEur 6.0 describes potassium alum as a granular powder, or
colorless, transparent, crystalline masses. The JP XV describes it as
colorless or white crystals or powder. Potassium alum is odorless
and has a slightly sweet, strongly astringent taste.
Chemical Properties
Aluminum is the most commonly available element in homes and workplaces. Aluminum
is readily available for human ingestion through the use of food additives, antacids,
buffered aspirin, astringents, nasal sprays, and antiperspirants; from drinking water;
from automobile exhaust and tobacco smoke; and from using aluminum foil, aluminum
cookware, cans, ceramics, and fi reworks. Workers who are at risk for toxicity are those
in refi neries, foundries and also welders and grinders.
Chemical Properties
white to colourless crystals
Uses
Buffer; floccculating reagent.
Uses
Aluminium potassium sulfate dodecahydrate is a white crystal that is soluble in water. It is made by roasting alunite in a furnace and then harvesting the product by crystallization. Aluminium potassium sulfate dodecahydrate was used to harden gelatin emulsions by introduction, by bathing the exposed plates prior to development, or by use in the fixing bath. Mixed with citric or other acids, it was also used as a clearing bath to remove developer stains in negatives.
Uses
potassium alum (alum) is a cosmetic astringent.
Definition
ChEBI: A hydrate resulting from the the formal combination of anhydrous potassium aluminium sulfate with 12 mol eq. of water.
Definition
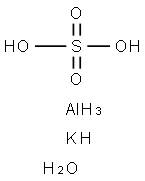
A group of double salts withthe formula A
2SO
4.B
2(SO
4)
3.24H
2O,where A is a monovalent metal and Ba trivalent metal. The original examplecontains potassium and aluminium(called potash alum orsimply alum); its formula is oftenwritten AlK(SO
4)
2.12H
2O (aluminiumpotassium sulphate-12-water). Ammoniumalum is AlNH
4(SO
4)
2.12H
2O,chrome alum is KCr(SO
4)
2.12H
2O, etc.The alums are isomorphous and canbe made by dissolving equivalentamounts of the two salts in waterand recrystallizing.
Production Methods
Potassium alum is manufactured by treating bauxite with sulfuric
acid and then potassium sulfate. Alternatively, aluminum sulfate is
reacted with potassium sulfate.
General Description
Aluminum potassium sulfate dodecahydrate is an inorganic sulfate salt of aluminum and potassium. Linear growth rates of (100), (111) and (110) faces of its crystals have been investigated.
Health Hazard
Prolonged periods of exposure to aluminum and dust cause symptoms of toxicity that
include, but are not limited to, coughing, wheezing, shortness of breath, memory loss,
learning diffi culty, loss of coordination, disorientation, mental confusion, colic, heartburn,
fl atulence, and headaches. Chronic exposures to alumina dust cause irritation to the eyes,
reaction suitability
reagent type: catalyst
core: aluminum
Pharmaceutical Applications
Potassium alum precipitates proteins and is a powerful astringent.
The ability to precipitate proteins is utilized in the manufacture of
vaccines, where purified proteins are coprecipitated with and
adsorbed onto potassium alum.
Potassium alum is often included in preparations used as
mouthwashes or gargles and in dermatological preparations, and
it may be used as a topical hemostatic, either as a solid or as a
solution. Intravesical instillation of potassium alum, typically as a
1% solution, has been used for hemorrhagic cystitis.
Safety
Potassium alum is often included in preparations used as
mouthwashes or gargles and in dermatological preparations.
Large doses of potassium alum act as an irritant and may be
corrosive; gum necrosis and gastrointestinal hemorrhage have
occurred. Acute encephalopathy has been reported following
bladder irrigation with alum solutions in the treatment of bladder
hemorrhage. Anecdotal evidence suggests that this practice should
be avoided for patients with renal insufficiency.
storage
Store in a cool, dry place in tightly closed containers. Stable under
normal temperatures and pressures. When kept for a long time at
60–65°C (or over sulfuric acid) potassium alum dodecahydrate
loses water, which is reabsorbed on exposure to air. It becomes
anhydrous at about 200°C.
Purification Methods
Crystallise it from weak aqueous H2SO4 (ca 0.5mL/g). Its solubility (%) in H2O is 5.7 (0o), 12.0 (20o) and 136.9 (100o).
Incompatibilities
Potassium alum is incompatible with strong oxidizing agents,
aluminum, copper, steel, and zinc. When it is dispensed in powders
with phenol, salicylates, or tannic acid, gray or green colors may be
developed owing to traces of iron in the alum.
Precautions
Aminocarb decomposes on heating and produces toxic fumes, irritating fumes, and oxides
of nitrogen. During handling of aminocarb workers should wear protective clothing,
gloves, face shield, breathing protection and avoid open flames.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database
(vaginal; suppository). Included in medicines licensed in the UK.