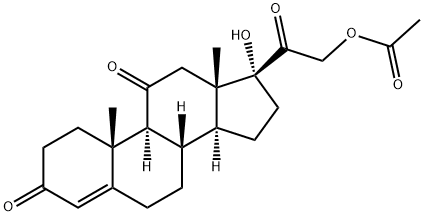
Cortison-21-acetat
Bezeichnung:Cortison-21-acetat
CAS-Nr50-04-4
Englisch Name:Cortisone acetate
CBNumberCB9277612
SummenformelC23H30O6
Molgewicht402.48
MOL-Datei50-04-4.mol
Synonyma
Cortison-21-acetat
Cortison-21-acetat physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 237-240 °C(lit.) |
| alpha | D25 +164° (c = 0.5 in acetone); D25 +208 to +217° (dioxane) |
| Siedepunkt | 577.3±50.0 °C(Predicted) |
| Dichte | 1.26±0.1 g/cm3(Predicted) |
| Brechungsindex | 212 ° (C=1, MeOH) |
| storage temp. | 2-8°C |
| Löslichkeit | Practically insoluble in water, freely soluble in methylene chloride, soluble in dioxan, sparingly soluble in acetone, slightly soluble in ethanol (96 per cent) and in methanol. |
| Aggregatzustand | Solid |
| pka | 12.32±0.60(Predicted) |
| Farbe | White to Off-White |
| Wasserlöslichkeit | 19mg/L(25 ºC) |
| Merck | 14,2539 |
| BRN | 2067543 |
| Stabilität | Stable. Incompatible with strong oxidizing agents. |
| CAS Datenbank | 50-04-4(CAS DataBase Reference) |
| EPA chemische Informationen | Cortisone acetate (50-04-4) |

