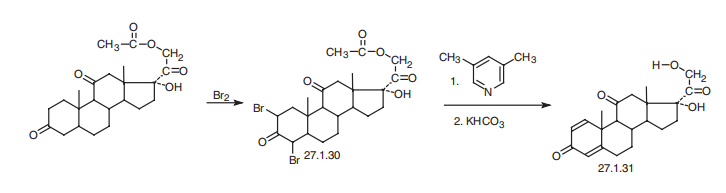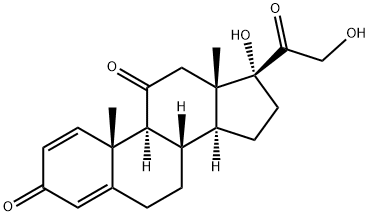
Prednison
Bezeichnung:Prednison
CAS-Nr53-03-2
Englisch Name:Prednisone
CBNumberCB6716222
SummenformelC21H26O5
Molgewicht358.43
MOL-Datei53-03-2.mol
Synonyma
Prednison
Prednison physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 236-238 °C(lit.) |
| alpha | 169 º (c=0.5, dioxane) |
| Siedepunkt | 410.86°C (rough estimate) |
| Dichte | 1.1121 (rough estimate) |
| Brechungsindex | 170 ° (C=0.5, Dioxane) |
| Flammpunkt | >200℃ |
| storage temp. | 2-8°C |
| Löslichkeit | Practically insoluble in water, slightly soluble in ethanol (96 per cent) and in methylene chloride. It shows polymorphism (5.9). |
| Aggregatzustand | Solid |
| pka | 12.36±0.60(Predicted) |
| Farbe | White to Off-White |
| Wasserlöslichkeit | 115mg/L(25 ºC) |
| Merck | 7722 |
| BRN | 2065301 |
| BCS Class | 1? |
| Stabilität | Stable. Incompatible with strong oxidizing agents. |
| CAS Datenbank | 53-03-2(CAS DataBase Reference) |
| IARC | 3 (Vol. 26, Sup 7) 1987 |
| Kennzeichnung gefährlicher | Xn |
| S-Sätze: | 36/37/39-45-26-16 |
| Giftige Stoffe Daten | 53-03-2(Hazardous Substances Data) |