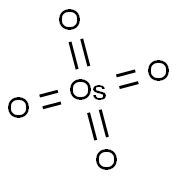
Osmiumtetraoxid
Bezeichnung:Osmiumtetraoxid
CAS-Nr20816-12-0
Englisch Name:Osmium tetroxide
CBNumberCB0853316
SummenformelO4Os
Molgewicht254.23
MOL-Datei20816-12-0.mol
Synonyma
Osmiumtetraoxid
Osmiums?ureanhydrid
Osmium(VIII)-oxid
Osmiumtetraoxid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 40°C |
| Siedepunkt | 130 °C |
| Dichte | 1.04 |
| chüttdichte | 1500kg/m3 |
| Dampfdichte | 8.8 (vs air) |
| Dampfdruck | 7 mmHg at 20 °C |
| Flammpunkt | -40 °C |
| storage temp. | 2-8°C |
| Löslichkeit | Soluble in alcohol, ether, chloroform, benzene, ammonium hydroxide, phosphorus oxychloride and carbon tetrachloride |
| Aggregatzustand | Solution |
| Farbe | White to yellow,or lemon yellow |
| Wichte | 5.10 |
| Geruch (Odor) | Acrid, chlorine-like odor detectable at 2 ppm (20 mg/m3) |
| PH | 6 (20°C, 25g/L in H2O) |
| Wasserlöslichkeit | Soluble in chloroform, alcohol and ethers.Soluble in water, organic solvents, benzene, alcohol, ether, ammonium hydroxide, phosphorus oxychloride and carbon tetrachloride. |
| Sensitive | Air Sensitive |
| Merck | 14,6893 |
| crystal system | Monoclinic |
| Kennzeichnung gefährlicher | O,T,C,T+,F,Xi,Xn |
| R-Sätze: | 8-23/24/25-40-34-26/27/28-42/43-11-67-66-36/37/38-19-36/38-36/37 |
| S-Sätze: | 17-26-27-36/37/39-45-7/9-36/37-16-28-23 |
| RIDADR | UN 3175 4.1/PG 2 |
| OEB | E |
| OEL | TWA: 0.002 mg/m3 (0.0002 ppm), STEL: 0.006 mg/m3 (0.0006 ppm) |
| WGK Germany | 3 |
| RTECS-Nr. | RN1140000 |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | I |
| HS Code | 28439090 |
| Giftige Stoffe Daten | 20816-12-0(Hazardous Substances Data) |
| Toxizität | LD50 oral (rat) 14 mg/kg LCLO inhal (rat) 40 ppm (4 h) PEL (OSHA) 0.0002 ppm (0.002 mg/m3) TLV-TWA (ACGIH) 0.0002 ppm (0.002 mg/m3) STEL (ACGIH) 0.0006 ppm (0.006 mg/m3) |
| IDLA | 1 mg Os/m3 |


