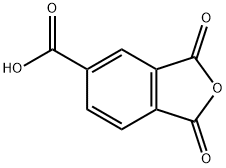
Benzol-1,2,4-tricarbonsure-1,2-anhydrid
Bezeichnung:Benzol-1,2,4-tricarbonsure-1,2-anhydrid
CAS-Nr552-30-7
Englisch Name:Trimellitic Anhydride
CBNumberCB5293056
SummenformelC9H4O5
Molgewicht192.13
MOL-Datei552-30-7.mol
Synonyma
Benzol-1,2,4-tricarbonsure-1,2-anhydrid
1,2,4-Benzoltricarbons?ureanhydrid
1,3-Dihydro-1,3-dioxo-5-isobenzofurancarbons?ure
Benzol-1,2,4-tricarbonsure-1,2-anhydrid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 163-166 °C (lit.) |
| Siedepunkt | 390 °C |
| Dichte | 1.54 |
| Dampfdichte | 6.6 (vs air) |
| Dampfdruck | <0.01 mm Hg ( 20 °C) |
| Brechungsindex | 1.4717 (estimate) |
| Flammpunkt | 227 °C |
| storage temp. | Store below +30°C. |
| Löslichkeit | 24.4g/l (decomposition) |
| pka | 3.11±0.20(Predicted) |
| Aggregatzustand | Flakes |
| Farbe | White to off-white |
| PH | 2 (21g/l, H2O, 20℃) |
| Explosionsgrenze | 1-7%(V) |
| Wasserlöslichkeit | DECOMPOSES |
| Sensitive | Moisture Sensitive |
| Merck | 14,9703 |
| BRN | 9394 |
| Kennzeichnung gefährlicher | Xn |
| R-Sätze: | 37-41-42/43 |
| S-Sätze: | 22-26-36/37/39 |
| OEB | D |
| OEL | TWA: 0.005 ppm (0.04 mg/m3) Should be handled in the workplace as an extremely toxic substance. |
| WGK Germany | 1 |
| RTECS-Nr. | DC2050000 |
| F | 10-21 |
| TSCA | Yes |
| HS Code | 29173980 |
| Giftige Stoffe Daten | 552-30-7(Hazardous Substances Data) |
| Toxizität | LD50 orally in Rabbit: > 2730 mg/kg LD50 dermal Rabbit 23000 mg/kg |



