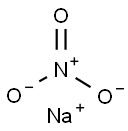
Natriumnitrat
Bezeichnung:Natriumnitrat
CAS-Nr7631-99-4
Englisch Name:Sodium nitrate
CBNumberCB8854258
SummenformelNNaO3
Molgewicht84.99
MOL-Datei7631-99-4.mol
Synonyma
Natriumnitrat
Chilesalpeter
Natronsalpeter
Natriumnitrat physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 306 °C (dec.) (lit.) |
| Siedepunkt | 380 °C |
| Dichte | 1.1 g/mL at 25 °C |
| chüttdichte | 1200kg/m3 |
| Brechungsindex | 1.526 |
| storage temp. | Store at RT. |
| Löslichkeit | H2O: 1 M at 20 °C, clear, colorless |
| Aggregatzustand | Solid |
| Wichte | 2.261 |
| Farbe | White or colorless |
| Geruch (Odor) | Odorless |
| PH | 5.5-8.0 (50g/l, H2O, 20℃) |
| Flame Color | Orange |
| Wasserlöslichkeit | 900 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8647 |
| crystal system | Three sides |
| Space group | R3c |
| Kennzeichnung gefährlicher | O,Xn,Xi,C |
| R-Sätze: | 8-22-36/37/38-36/38-34-36 |
| S-Sätze: | 17-26-27-36/37/39-37/39-36-45 |
| RIDADR | UN 1498 5.1/PG 3 |
| WGK Germany | 1 |
| RTECS-Nr. | WC5600000 |
| F | 3 |
| TSCA | Yes |
| HazardClass | 5.1 |
| PackingGroup | III |
| HS Code | 31025090 |
| Giftige Stoffe Daten | 7631-99-4(Hazardous Substances Data) |
| Toxizität | LD50 orally in rabbits: 1.955 g anion/kg (Dollahite, Rowe) |


