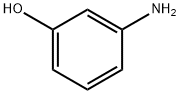
3-Aminophenol
Bezeichnung:3-Aminophenol
CAS-Nr591-27-5
Englisch Name:3-Aminophenol
CBNumberCB8853903
SummenformelC6H7NO
Molgewicht109.13
MOL-Datei591-27-5.mol
Synonyma
3-Aminophenol
3-Aminophenol physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 120-124 °C (lit.) |
| Siedepunkt | 164 °C/11 mmHg (lit.) |
| Dichte | 0.99 |
| chüttdichte | 700kg/m3 |
| Dampfdruck | 0.019Pa at 20℃ |
| Brechungsindex | 1.5444 (estimate) |
| Flammpunkt | 155 °C |
| storage temp. | 2-8°C |
| Löslichkeit | 26g/l |
| Colour Index | 76545 |
| Aggregatzustand | Crystalline Powder |
| pka | 4.37, 9.82(at 20℃) |
| Farbe | White to pinkish or light gray |
| Geruch (Odor) | odorless |
| PH | 6.8 (10g/l, H2O, 20℃) |
| Wasserlöslichkeit | 35 g/L (20 ºC) |
| Merck | 14,460 |
| BRN | 636059 |
| Kennzeichnung gefährlicher | Xn,N |
| R-Sätze: | 20/22-51/53 |
| S-Sätze: | 28-61-28A |
| RIDADR | UN 2512 6.1/PG 3 |
| WGK Germany | 2 |
| RTECS-Nr. | SJ4900000 |
| Selbstentzündungstemperatur | >600°C |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | III |
| HS Code | 29222900 |
| Giftige Stoffe Daten | 591-27-5(Hazardous Substances Data) |
| Toxizität | LD50 i.p. in mice: 4.5 mg/20g (Koelzer, Giesen) |


