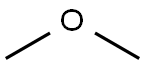
Dimethyl ether
Bezeichnung:Dimethyl ether
CAS-Nr115-10-6
Englisch Name:Dimethyl ether
CBNumberCB8765901
SummenformelC2H6O
Molgewicht46.07
MOL-Datei115-10-6.mol
Synonyma
Methylether
Oxybismethan
Holzether
Dimethyl ether physikalisch-chemischer Eigenschaften
| Schmelzpunkt | −141 °C(lit.) |
| Siedepunkt | −24.8 °C(lit.) |
| Dichte | 1.617 |
| Dampfdichte | 1.62 (vs air) |
| Dampfdruck | >760 mm Hg ( 25 °C) |
| Brechungsindex | 1.2984 |
| Flammpunkt | -41°C |
| Löslichkeit | Soluble in acetone, chloroform, ethanol (95%), ether, and 1 in 3 parts of water. Dimethyl ether is generally miscible with water, nonpolar materials, and some semipolar materials. For pharmaceutical aerosols, ethanol (95%) is the most useful cosolvent. Glycols, oils, and other similar materials exhibit varying degrees of miscibility with dimethyl ether. |
| Aggregatzustand | gas |
| Geruch (Odor) | Chloroform-like; sweet. |
| Odor Threshold | 500ppm |
| Explosionsgrenze | 27% |
| Wasserlöslichkeit | soluble |
| Merck | 6071 |
| BRN | 1730743 |
| Dielectric constant | 5.0(25℃) |
| Stabilität | Stable. Extremely flammable. Note low flash point. May form explosive mixtures with air. May form peroxides during prolonged storage. |
| LogP | 0.07 at 25℃ |
| Kennzeichnung gefährlicher | F+ |
| R-Sätze: | 12 |
| S-Sätze: | 9-16-33 |
| RIDADR | UN 1033 2.1 |
| WGK Germany | 1 |
| RTECS-Nr. | PM4780000 |
| F | 4.5-31 |
| Selbstentzündungstemperatur | 662 °F |
| HazardClass | 2.1 |
| HS Code | 2909199090 |
| Giftige Stoffe Daten | 115-10-6(Hazardous Substances Data) |


