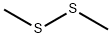
Dimethyldisulfid
Bezeichnung:Dimethyldisulfid
CAS-Nr624-92-0
Englisch Name:Dimethyl disulfide
CBNumberCB8229876
SummenformelC2H6S2
Molgewicht94.2
MOL-Datei624-92-0.mol
Synonyma
Dimethyldisulfid
Methyldisulfid
Methyldithiomethan
Dimethyldisulfid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | -85 °C |
| Siedepunkt | 109 °C(lit.) |
| Dichte | 1.0625 |
| Dampfdichte | 3.24 (vs air) |
| Dampfdruck | 22 mm Hg ( 20 °C) |
| FEMA | 3536 | DIMETHYL DISULFIDE |
| Brechungsindex | n |
| Flammpunkt | 76 °F |
| storage temp. | Flammables area |
| Löslichkeit | 2.7g/l |
| Aggregatzustand | Liquid |
| Wichte | 1.0647 (20/4℃) |
| Farbe | Clear yellow |
| Geruch (Odor) | at 0.10 % in propylene glycol. sulfurous vegetable cabbage onion |
| Geruchsart | sulfurous |
| Biologische Quelle | rabbit |
| Odor Threshold | 0.0022ppm |
| Explosionsgrenze | 1.1-16.1%(V) |
| Kennzeichnung gefährlicher | F,Xn,N,T+ |
| R-Sätze: | 11-20/22-36-51/53-36/37/38-26-22-36/37 |
| S-Sätze: | 26-61-45-38-36/37/39-28A-16-60-57-39-29 |
| RIDADR | UN 2381 3/PG 2 |
| WGK Germany | 2 |
| RTECS-Nr. | JO1927500 |
| Selbstentzündungstemperatur | 304 °C |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | II |
| HS Code | 29309070 |
| Giftige Stoffe Daten | 624-92-0(Hazardous Substances Data) |
| Toxizität | LD50 orally in Rabbit: 290 - 500 mg/kg |




