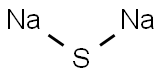
Dinatriumsulfid
Bezeichnung:Dinatriumsulfid
CAS-Nr1313-82-2
Englisch Name:Sodium sulfide
CBNumberCB3429046
SummenformelNa2S
Molgewicht78.04
MOL-Datei1313-82-2.mol
Synonyma
Dinatriumsulfid
Natriummonosulfid
Dinatriumsulfd
Dinatriumsulfid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 950 °C(lit.) | ||||||||||||||
| Dichte | 1.86 g/mL at 25 °C(lit.) | ||||||||||||||
| storage temp. | Refrigerator (+4°C) | ||||||||||||||
| Löslichkeit | H2O: 0.1 g/mL, clear, colorless | ||||||||||||||
| Aggregatzustand | flakes | ||||||||||||||
| Farbe | Yellow | ||||||||||||||
| Wichte | 1.856 | ||||||||||||||
| Geruch (Odor) | Rotten egg odor | ||||||||||||||
| Wasserlöslichkeit | 186 g/L (20 ºC) | ||||||||||||||
| Sensitive | Hygroscopic | ||||||||||||||
| Crystal Structure | Reverse CaF2 type | ||||||||||||||
| crystal system | Cube | ||||||||||||||
| Merck | 14,8681 | ||||||||||||||
| Space group | Fm3m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Dielectric constant | 5.0(Ambient) | ||||||||||||||
| Stabilität | Spontaneously flammable. Incompatible with acids, metals, oxidizing agents. Contact with acid liberates toxic gas. Fine dust/air mixtures are explosive. Hygroscopic. | ||||||||||||||
| InChIKey | CXPWOVUZRAFMDA-UHFFFAOYSA-N |
| Kennzeichnung gefährlicher | C,N,Xn |
| R-Sätze: | 31-34-50-22 |
| S-Sätze: | 26-45-61-36/37/39 |
| RIDADR | UN 1849 8/PG 2 |
| WGK Germany | 2 |
| RTECS-Nr. | WE1905000 |
| F | 3-8-9-23 |
| TSCA | Yes |
| HazardClass | 4.2 |
| PackingGroup | II |
| HS Code | 28301010 |
| Giftige Stoffe Daten | 1313-82-2(Hazardous Substances Data) |




