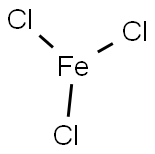
Eisentrichlorid
Bezeichnung:Eisentrichlorid
CAS-Nr7705-08-0
Englisch Name:Ferric chloride
CBNumberCB5444364
SummenformelCl3Fe
Molgewicht162.2
MOL-Datei7705-08-0.mol
Synonyma
Eisentrichlorid
Eisenchlorid
Ferrichlorid
Eisentrichlorid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 304 °C(lit.) |
| Siedepunkt | 316 °C |
| Dichte | 2,804 g/cm3 |
| chüttdichte | 1000kg/m3 |
| Dampfdichte | 5.61 (vs air) |
| Dampfdruck | 1 mm Hg ( 194 °C) |
| Brechungsindex | n20/D1.414 |
| Flammpunkt | 316°C |
| storage temp. | Store below +30°C. |
| Löslichkeit | H2O: soluble |
| Aggregatzustand | powder |
| Farbe | Yellow |
| Wichte | 2.804 |
| PH | 1 (200g/l, H2O, 20℃) |
| Wasserlöslichkeit | 920 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Crystal Structure | BiI3 type |
| crystal system | Three sides |
| Kennzeichnung gefährlicher | C,Xn,Xi |
| R-Sätze: | 41-38-22-34-37/38-10-36 |
| S-Sätze: | 26-39-45-36/37/39 |
| RIDADR | UN 2582 8/PG 3 |
| WGK Germany | 1 |
| RTECS-Nr. | LJ9100000 |
| TSCA | Yes |
| HS Code | 2827 39 20 |
| HazardClass | 8 |
| PackingGroup | III |
| Giftige Stoffe Daten | 7705-08-0(Hazardous Substances Data) |
| Toxizität | LD50 orally in Rabbit: 316 mg/kg |




