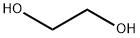
Ethandiol
Bezeichnung:Ethandiol
CAS-Nr107-21-1
Englisch Name:Ethylene glycol
CBNumberCB7852707
SummenformelC2H6O2
Molgewicht62.07
MOL-Datei107-21-1.mol
Synonyma
Ethan-1,2-diol
Ethandiol
1,2-Ethandiol
1,2-Dihydroxyethan
Ethandiol physikalisch-chemischer Eigenschaften
| Schmelzpunkt | -13 °C (lit.) |
| Siedepunkt | 195-198 °C |
| Dichte | 1.113 g/mL at 25 °C (lit.) |
| Dampfdichte | 2.1 (vs air) |
| Dampfdruck | 0.08 mm Hg ( 20 °C) |
| Brechungsindex | n |
| Flammpunkt | 230 °F |
| storage temp. | 2-8°C |
| Löslichkeit | water: miscible |
| Aggregatzustand | Viscous Liquid |
| pka | 14.22(at 25℃) |
| Farbe | blue |
| Relative polarity | 0.79 |
| PH | 6-7.5 (100g/l, H2O, 20℃) |
| Geruch (Odor) | Odorless |
| Explosionsgrenze | 3.2%(V) |
| Wasserlöslichkeit | miscible |
| FreezingPoint | -11.5℃ |
| Sensitive | Hygroscopic |
| maximale Wellenlänge (λmax) | λ: 260 nm Amax: ≤0.03 λ: 280 nm Amax: ≤0.01 |
| Merck | 14,3798 |
| BRN | 505945 |
| Expositionsgrenzwerte | Ceiling limit in air for vapor and mist 50 ppm (~125 mg/m3) (ACGIH); TWA 10 mg/m3 (particulates) (MSHA). |
| Dielectric constant | 37.0(20℃) |
| LogP | -1.36 at 25℃ |
| Oberflächenspannung | 47.7mN/m at 20°C |
| CAS Datenbank | 107-21-1(CAS DataBase Reference) |
| NIST chemische Informationen | 1,2-Ethanediol(107-21-1) |
| EPA chemische Informationen | Ethylene glycol (107-21-1) |
| Kennzeichnung gefährlicher | Xn |
| R-Sätze: | 22-36-41 |
| S-Sätze: | 26-39-36/37/39 |
| WGK Germany | 3 |
| RTECS-Nr. | KW2975000 |
| Selbstentzündungstemperatur | 752 °F |
| TSCA | Yes |
| HS Code | 29053100 |
| Giftige Stoffe Daten | 107-21-1(Hazardous Substances Data) |
| Toxizität | LD50 in rats, guinea pigs (g/kg): 8.54, 6.61 orally (Smyth); in mice (ml/kg): 13.79 orally (Bornmann) |
Gefahreninformationscode (GHS)
-
Bildanzeige (GHS)


-
Alarmwort
Warnung
-
Gefahrenhinweise
H302:Gesundheitsschädlich bei Verschlucken.
H373:Kann die Organe schädigen bei längerer oder wiederholter Exposition.
-
Sicherheit
P260:Dampf/Aerosol/Nebel nicht einatmen.
P264:Nach Gebrauch gründlich waschen.
P264:Nach Gebrauch gründlich waschen.
P270:Bei Gebrauch nicht essen, trinken oder rauchen.
P301+P312:BEI VERSCHLUCKEN: Bei Unwohlsein GIFTINFORMATIONSZENTRUM/Arzt/... (geeignete Stelle für medizinische Notfallversorgung vom Hersteller/Lieferanten anzugeben) anrufen.
P314:Bei Unwohlsein ärztlichen Rat einholen / ärztliche Hilfe hinzuziehen.
P501:Inhalt/Behälter ... (Entsorgungsvorschriften vom Hersteller anzugeben) zuführen.
Ethylene glycol Chemische Eigenschaften,Einsatz,Produktion Methoden
-
ERSCHEINUNGSBILD
GERUCHLOSE, FARBLOSE, VISKOSE, HYGROSKOPISCHE FLüSSIGKEIT. -
CHEMISCHE GEFAHREN
Beim Verbrennen Bildung giftiger Gase. Reagiert mit starken Oxidationsmitteln und starken Basen. -
ARBEITSPLATZGRENZWERTE
TLV: 100 mg/m?(als STEL, ceiling); Krebskategorie A4 (nicht klassifizierbar als krebserzeugend für den Menschen); (ACGIH 2005).
MAK: 10 ppm, 26 mg/m? Spitzenbegrenzung: überschreitungsfaktor I(2); Hautresorption; Schwangerschaft: Gruppe C; (DFG 2005)
-
AUFNAHMEWEGE
Aufnahme in den Körper durch Inhalation und über die Haut. -
INHALATIONSGEFAHREN
Beim Verdampfen bei 20°C tritt langsam eine gesundheitsschädliche Kontamination der Luft ein. -
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION:
Die Substanz reizt die Augen und die Atemwege. Möglich sind Auswirkungen auf Nieren und Zentralnervensystem mit nachfolgenden Nierenschäden und Hirnverletzung. Exposition kann Bewusstseinstrübung verursachen. -
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Möglich sind Auswirkungen auf das Zentralnervensystem mit nachfolgenden krankhaften Augenbewegungen (Nystagmus). -
LECKAGE
Ausgelaufene Flüssigkeit möglichst in abdichtbaren Behältern sammeln. Reste mit viel Wasser wegspülen. Persönliche Schutzausrüstung: Atemschutzfilter für organische Gase und Dämpfe. -
R-Sätze Betriebsanweisung:
R22:Gesundheitsschädlich beim Verschlucken.
R36:Reizt die Augen. -
S-Sätze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren. -
Aussehen Eigenschaften
C2H6O2; 1,2-Ethandiol, Glycol. Farblose, fast geruchlose Flüssigkeit. -
Gefahren für Mensch und Umwelt
Gesundheitsschädlich beim Verschlucken und Einatmen der Dämpfe.
Nicht mit Aluminium, Chromylchlorid, Alkalihydroxiden, Perchlorsäure und starken Oxidationsmitteln in Berührung bringen.
LD50 (oral, Ratte): 4700 mg/kg -
Schutzmaßnahmen und Verhaltensregeln
Schutzhandschuhe als kurzzeitiger Spritzschutz. -
Verhalten im Gefahrfall
Dämpfe nicht einatmen.
Mit flüssigkeitsbindendem Material, z. B. Rench Rapid aufnehmen. Der Entsorgung zuführen. Nachreinigen.
Wasser, Schaum.
Brennbar. Dämpfe schwerer als Luft. Mit Luft Bildung explosionsfähiger Gemische möglich. -
Erste Hilfe
Nach Hautkontakt: Mit reichlich Wasser abwaschen.
Nach Augenkontakt: Mit reichlich Wasser bei geöffnetem Lidspalt mindestens 10 Minuten ausspülen. Augenarzt hinzuziehen. Augenarzt hinzuziehen.
Nach Einatmen: Frischluft.
Nach Verschlucken: Reichlich Wasser trinken. Erbrechen auslösen. Arzt hinzuziehen.
Nach Kleidungskontakt: Kontaminierte Kkleidung sofort entfernen.
Ersthelfer: siehe gesonderten Anschlag -
Sachgerechte Entsorgung
Als halogenfreie, organische Lösemittelabfälle. -
Beschreibung
Ethylene glycol was first synthesized in 1859; however, it did not become a public health concern until after World War II. In fact, the first published series of deaths from ethylene glycol consumption involved 18 soldiers who drank antifreeze as a substitute for ethanol. Despite the early recognition that patients who drank ethanol in addition to ethylene glycol had prolonged survival when compared to those drinking ethylene glycol alone, antidotal treatment of ethylene glycol toxicity with ethanol was not evaluated until the 1960s. Today, ethylene glycol poisoning continues to be a public health problem, particularly in the southeastern United States. In 2009, US poison control centers received 5282 calls about possible ethylene glycol exposures, and the toxicology community believes these exposures are underreported. -
Chemische Eigenschaften
Ethylene glycol is a colorless, viscous, hydroscopic liquid with a sweetish taste. Often colored fluorescent yellow-green when used in automotive antifreeze. Ethylene glycol is odorless and does not provide any warning of inhalation exposure to hazardous concentrations. The Odor Threshold in air is 25 ppm. -
Chemische Eigenschaften
Ethylene glycol,CH20HCH20H, also known as glycol,ethylene alcohol, glycol alcohol, and dihydric alcohol, is a colorless liquid. It is soluble in water and in alcohol. Ethyleneglycol has a low freezing point,-25°C (-13 OF), and is widely used as an antifreeze in automobiles and in hydraulic fluids. It is used as a solvent for nitrocellulose and in the manufacture of acrylonitrile, dynamites, and resins. -
Verwenden
Antifreeze in cooling and heating systems. In hydraulic brake fluids and de-icing solutions. Industrial humectant. Ingredient of electrolytic condensers (where it serves as solvent for boric acid and borates). Solvent in the paint and plastics industries. In the formulation of printers' inks, stamp pad inks, ball-point pen ink. Softening agent for cellophane. Stabilizer for soybean foam used to extinguish oil and gasoline fires. In the synthesis of safety explosives, glyoxal, unsatd ester type alkyd resins, plasticizers, elastomers, synthetic fibers (Terylene, Dacron), and synthetic waxes. To create artificial smoke and mist for theatrical uses. -
Verwenden
Reagent typically used in cyclocondensation reactions with aldehydes1 and ketones1,2 to form 1,3-dioxolanes. -
Verwenden
Ethylene glycol is used as an antifreeze inheating and cooling systems (e.g., automobileradiators and coolant for airplane motors).It is also used in the hydraulic brake fluids;as a solvent for paints, plastics, and inks; as a softening agent for cellophane; and in themanufacture of plasticizers, elastomers, alkydresins, and synthetic fibers and waxes. -
Vorbereitung Methode
Historically, ethylene glycol has been manufactured by hydrolyzing ethylene oxide. Presently, it is also produced commercially by oxidizing ethylene in the presence of acetic acid to form ethylene diacetate, which is hydrolyzed to the glycol, and acetic acid is recycled in the process . -
Definition
ChEBI: A 1,2-glycol compound produced via reaction of ethylene oxide with water. -
synthetische
Ethylene glycol is prepared by the hydration of ethylene oxide:

This reaction is carried out in a manner comparable to that described for the preparation of propylene glycol from propylene oxide . Ethylene glycol is a colourless liquid, b.p. 197??C. -
Reaktionen
Glycol reacts (1) with sodium to form sodium glycol, CH2OH · CH2ONa, and disodium glycol, CH2ONa·CH2ONa; (2) with phosphorus pentachloride to form ethylene dichloride, CH2Cl·CH2Cl (3) with carboxy acids to form mono- and disubstituted esters, e.g., glycol monoacetate, CH2OH·CH2OOCCH3, glycol diacetate, CH3COOCH2 · CH2OOCCH3; (4) with nitric acid (with sulfuric acid), to form glycol mononitrate, CH2OH·CH2ONO2, glycol dinitrate, CH2ONO2 · CH2ONO2; (5) with hydrogen chloride, heated, to form glycol chlorohydrin (ethylene chlorohydrin, CH2OH·CHCl); (6) upon regulated oxidation to form glycollic aldehyde, CH2OH·CHO, glyoxal, CHO · CHO, glycollic acid, CH2OH·COOH, glyoxalic acid, CHO·COOH, oxalic acid, COOH·COOH. -
Allgemeine Beschreibung
Ethylene glycol is a clear, colorless syrupy liquid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Since Ethylene glycol is a liquid Ethylene glycol can easily penetrate the soil and contaminate groundwater and nearby streams. -
Reaktivität anzeigen
Mixing Ethylene glycol in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: chlorosulfonic acid, oleum, sulfuric acid, [NFPA 1991]. -
Hazard
Questionable carcinogen. Toxic by ingestion and inhalation. Lethal dose reported to be 100 cc. -
Health Hazard
The acute inhalation toxicity of 1,2-ethanediolis low. This is due to its low vaporpressure, 0.06 torr at 20°C (68°F). Its saturationconcentration in air at 20°C (68°F)is 79 ppm and at 25°C (77°F) is 131 ppm(ACGIH 1986). Both concentrations exceedthe ACGIH ceiling limit in air, which is50 ppm. In humans, exposure to its mist orvapor may cause lacrimation, irritation ofthroat, and upper respiratory tract, headache,and a burning cough. These symptoms maybe manifested from chronic exposure toabout 100 ppm for 8 hours per day for severalweeks.
The acute oral toxicity of 1,2-ethanediol islow to moderate. The poisoning effect, however,is much more severe from ingestionthan from inhalation. Accidental ingestion of80–120 mL of this sweet-tasting liquid canbe fatal to humans. The toxic symptoms inhumans may be excitement or stimulation,followed by depression of the central nervoussystem, nausea, vomiting, and drowsiness,which may, in the case of severe poisoning,progress to coma, respiratory failure, anddeath. When rats were administered sublethaldoses over a long period, there was depositionof calcium oxalate in tubules, causinguremic poisoning.
LD50 value, oral (rats): 4700 mg/kg
Ingestion of 1,2-ethanediol produced reproductiveeffects in animals, causing fetotoxicity, postimplantation mortality, andspecific developmental abnormalities. Mutagenictests proved negative. It tested negativeto the histidine reversion–Ames test. -
Health Hazard
Inhalation of vapor is not hazardous. Ingestion causes stupor or coma, sometimes leading to fatal kidney injury. -
Brandgefahr
Ethylene glycol is combustible. -
Flammability and Explosibility
Not classified -
Biochem/physiol Actions
Ethylene glycol is a low toxicity molecule and is used for embryo cryopreservation in many domestic animals.Ethylene glycol 5M solution is an additive screening solution of Additive Screening Kit. Additive Screen kit is designed to allow rapid and convenient evaluation of additives and their ability to influence the crystallization of the sample. The Additive Kit provides a tool for refining crystallization conditions. -
Sicherheitsprofil
Human poison by ingestion. (Lethal dose for humans reported to be 100 mL.) Moderately toxic to humans by an unspecified route. Moderately toxic experimentally by ingestion, subcutaneous, intravenous, and intramuscular routes. Human systemic effects by ingestion and inhalation: eye lachrymation, general anesthesia, headache, cough, respiratory stimulation, nausea or vomiting, pulmonary, kidney, and liver changes. If ingested it causes initial central nervous system stimulation followed by depression. Later, it causes potentially lethal kidney damage. Very toxic in particulate form upon inhalation. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. A skin, eye, and mucous membrane irritant. Combustible when exposed to heat or flame; can react vigorously with oxidants. Moderate explosion hazard when exposed to flame. Iptes on contact with chromium trioxide, potassium permanganate, and sodium peroxide. Mixtures with ammonium dichromate, silver chlorate, sodium chlorite, and uranyl nitrate ipte when heated to 100°C. Can react violently with chlorosulfonic acid, oleum, H2SO4, HClO4, and Pass. Aqueous solutions may ignite silvered copper wires that have an applied D.C. voltage. To fight fire, use alcohol foam, water, foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. -
mögliche Exposition
Ethylene glycol is used in antifreeze (especially as car radiator antifreeze) and in production of polyethylene terephthalate fibers and films; in hydraulic fluids; antifreeze and coolant mixtures for motor vehicles; electrolytic condensers; and heat exchangers. It is also used as a solvent and as a chemical intermediate for ethylene glycol dinitrate, glycol esters; resins, and for pharmaceuticals. -
Erste Hilfe
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, getmedical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit -
Environmental Fate
Ethylene glycol is considered an inert ingredient in pesticides. It typically enters the environment through waste streams after use of deicing products, where it is highly mobile in soil and contaminates groundwater. Ethylene glycol is considered ‘readily biodegradable.’ It biodegrades relatively quickly; its half-life (t1/2) is 2–12 days in soil.
Ethylene glycol is biodegraded in water under both aerobic and anaerobic conditions within a day to a few weeks. In the atmosphere, ethylene glycol photochemically degrades with a t1/2 of approximately 2 days. -
Solubility in organics
Miscible with water and alcohol, soluble in lower atifatic alcohols and ketones, Propylene glycol and Glycerin, poorly soluble in Hydrocarbons such as Terpenes as well as in Terpene alcohols, esters, etc. -
Lager
Color Code—Green: General storage may be used.Prior to working with this chemical you should be trainedon its proper handling and storage. Before entering confinedspace where this chemical may be present, check to makesure that an explosive concentration does not exist.Ethylene glycol must be stored to avoid contact with sulfuric acid since violent reactions occur. Store in tightly closedcontainers in a cool, well-ventilated area away from oxidizing agents (such as perchlorates, peroxides, permanganates,chlorates, and nitrates). -
Versand/Shipping
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required -
läuterung methode
It is very hygroscopic, and also likely to contain higher diols. Dry it with CaO, CaSO4, MgSO4 or NaOH and distil it under vacuum. Dry further by reaction with sodium under nitrogen, reflux for several hours and distil. The distillate is then passed through a column of Linde type 4A molecular sieves and finally distil under nitrogen, from more molecular sieves. Then fractionally distil it. [Beilstein 1 IV 2369.] -
Toxicity evaluation
Ethylene glycol has low toxicity but it is metabolized to a variety of toxic metabolites. Ethylene glycol and glycolaldehyde have an intoxicating effect on the central nervous system that can lead to ataxia, sedation, coma, and respiratory arrest similar to ethanol intoxication. However, the profound metabolic acidosis reported in toxicity is secondary to accumulation of acid metabolites, especially glycolic acid. The oxalic acid metabolite complexes with calcium and precipitates as calcium oxalate crystals in the renal tubules, leading to acute renal injury. Further, oxalate’s ability to chelate calcium may cause clinically relevant serum hypocalcemia. -
Inkompatibilitäten
Reacts with sulfuric acid, oleum, chlorosulfonic acid; strong oxidizing agents; strong bases; chromium trioxide; potassium permanganate; sodium peroxide. Hygroscopic (i.e., absorbs moisture from the air) -
Filter für giftige stoffe
The ITSL for ethylene glycol is 1000 μg/m3 based on a 1 hour averaging time. -
Waste disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Alternatively, ethylene glycol can be recovered from polyester plant wastes
Ethylene glycol Upstream-Materialien And Downstream Produkte
Upstream-Materialien
1of2
Downstream Produkte
- 2,4-DIAMINO-6-MERCAPTOPYRIMIDINE
- 2-(2-Bromethyl)-1,3-dioxolan
- tussah silk fabric aftertreatment finishing agent
- 5-Amino-1,2,3,4-tetrahydrophtha-lazin-1,4-dion
- Oxalic acid dihydrate
- 2-Ethyl-5-methylthiophene
- 4-Formylphenylboronic acid
- 5-Chlorooxindole
- Polyester Filament
- PolyesterPolyol
- 3-BENZOYL PROPIOPHENONE
- Dye-fixing agent,no formaldehyde
- solid alcohol
- 1-Vinyl-2-pyrrolidon
- 6-Methyl-1,2,4-triazin-3,5-diol
- 21-Iodo-16-methylpregna-1,4,9(11)-trien-17-ol-3,20-dione
- 2,2'-[Ethylenbis(oxymethylen)]bisoxiran
- 1-Chlor-2-methylpropen
- 2,3-THIOPHENEDICARBOXALDEHYDE
- 4-(Hydroxymethyl)phenylboronic acid
- 1,4-Dioxacycloheptadecan-5,17-dion
- 2-(2,4-Dinitrophenoxy)ethanol
- Antifreeze
- 16,17-Epoxypregna-5,9(11)-diene-3,20-dione cyclic bis(1,2-ethanediyl acetal)
- Saponified soluble oil
- 16-Methylpregna-1,4,9(11)-trien-17-ol-3,20-dione
- 9-Bromo-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione-21-acetate
- 16-Methylpregna-4,9(11)-dien-17-ol-3,20-dione
- 17-Ethinyl-17-hydroxy-18-methylestra-5(10),9(11)-dien-3-one-3-ethylene ketal
- 17,21-Dihydroxy-16β-methylpregna-1,4,9(11)-trien-3,20-dion-21-acetat
1of8
Ethandiol Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(1415)Suppliers
Region
-
Shandong Deshang Chemical Co., Ltd.
Telefon +86-0531-8875-2665<br/>+8613153039501
E-Mail info@deshangchem.com
-
Shandong Zhishang New Material Co., Ltd.
Telefon +8617653113209
E-Mail sales002@sdzschem.com
-
Telefon +86-85511178;<br/>+86-85511178;
E-Mail peter68@ptchemgroup.com
-
Shanghai Bojing Chemical Co.,Ltd.
Telefon +86-86-02137122233<br/>+8613795318958
E-Mail bj1@bj-chem.com
-
Yujiang Chemical (Shandong) Co.,Ltd.
Telefon +8617736087130
E-Mail catherine@yjchem.com.cn
-
Telefon
E-Mail tp@aladdinsci.com
-
Shandong Dexiang International Trade Co., Ltd
Telefon +86-15662691337<br/>+86-15662695772
E-Mail 539942812@qq.com
-
SHANGHAI KEAN TECHNOLOGY CO., LTD.
Telefon +8613817748580
E-Mail cooperation@kean-chem.com
-
Hebei Chuanghai Biotechnology Co., Ltd
Telefon +86-15531157085<br/>+86-15531157085
E-Mail abby@chuanghaibio.com
-
Hebei Mujin Biotechnology Co.,Ltd
Telefon +86 13288715578<br/>+8613288715578
E-Mail sales@hbmojin.com
1of2
