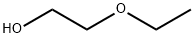
Ethylenglykolmonoethylether
Bezeichnung:Ethylenglykolmonoethylether
CAS-Nr110-80-5
Englisch Name:2-Ethoxyethanol
CBNumberCB6852821
SummenformelC4H10O2
Molgewicht90.12
MOL-Datei110-80-5.mol
Synonyma
2-Ethoxyethanol
Ethylenglykolmonoethylether
Monoethylglykolether
Ethylenglykolmonoethylether physikalisch-chemischer Eigenschaften
| Schmelzpunkt | -100 °C |
| Siedepunkt | 135 °C(lit.) |
| Dichte | 0.93 g/mL at 25 °C(lit.) |
| Dampfdichte | 3.1 (vs air) |
| Dampfdruck | 3.8 mm Hg ( 20 °C) |
| Brechungsindex | n |
| Flammpunkt | 107.6 °F |
| storage temp. | Store below +30°C. |
| Löslichkeit | water: miscible |
| pka | 14.44±0.10(Predicted) |
| Aggregatzustand | Liquid |
| Farbe | Clear, colorless |
| Geruch (Odor) | Sweetish; mild, pleasant, ethereal. |
| PH | 7 (20°C) |
| Odor Threshold | 0.58ppm |
| Explosionsgrenze | 1.8-15.7%(V) |
| Wasserlöslichkeit | miscible |
| FreezingPoint | -70℃ |
| Kennzeichnung gefährlicher | T |
| R-Sätze: | 60-61-10-20/21/22-20/22 |
| S-Sätze: | 53-45 |
| RIDADR | UN 1171 3/PG 3 |
| OEB | B |
| OEL | TWA: 0.5 ppm (1.8 mg/m3) [skin] |
| WGK Germany | 1 |
| RTECS-Nr. | KK8050000 |
| Selbstentzündungstemperatur | 460 °F |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | III |
| HS Code | 29094990 |
| Giftige Stoffe Daten | 110-80-5(Hazardous Substances Data) |
| Toxizität | Acute oral LD50 for guinea pigs 1,400 mg/kg, mice 2,451 mg/kg, rats 3,000 mg/kg, rabbits 3,100 mg/kg (quoted, RTECS, 1985). |
| IDLA | 500 ppm |



