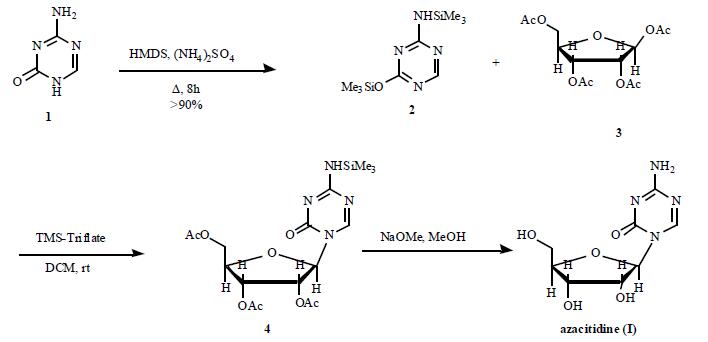
2-(β-D-Ribofuranosyl)-4-amino-1,3,5-triazin-2-on
Bezeichnung:2-(β-D-Ribofuranosyl)-4-amino-1,3,5-triazin-2-on
CAS-Nr320-67-2
Englisch Name:5-Azacytidine
CBNumberCB4383338
SummenformelC8H12N4O5
Molgewicht244.2
MOL-Datei320-67-2.mol
Synonyma
2-(β-D-Ribofuranosyl)-4-amino-1,3,5-triazin-2-on
2-(β-D-Ribofuranosyl)-4-amino-1,3,5-triazin-2-on physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 226-232 °C (dec.)(lit.) |
| alpha | 40 º (C=1, H2O 22 ºC) |
| Siedepunkt | 387.12°C (rough estimate) |
| Dichte | 1.4287 (rough estimate) |
| Brechungsindex | 1.6590 (estimate) |
| storage temp. | -20°C |
| Löslichkeit | Soluble in DMSO (up to 25 mg/ml), or in Water (up to 12 mg/ml). |
| pka | 13.46±0.70(Predicted) |
| Aggregatzustand | lyophilized powder |
| Farbe | White to Off-white |
| Biologische Quelle | synthetic (organic) |
| Wasserlöslichkeit | 0.5-1.0 g/100 mL at 21 ºC |
| Merck | 14,887 |
| BRN | 620461 |
| Stabilität | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20° for up to 1 month. |
| InChIKey | NMUSYJAQQFHJEW-WGDKFINWSA-N |
| LogP | -2.191 (est) |
| CAS Datenbank | 320-67-2(CAS DataBase Reference) |
| Kennzeichnung gefährlicher | T |
| R-Sätze: | 45-46-22 |
| S-Sätze: | 53-22-36/37/39-45 |
| WGK Germany | 3 |
| RTECS-Nr. | XZ3017500 |
| F | 10-23 |
| HS Code | 29349990 |
| Giftige Stoffe Daten | 320-67-2(Hazardous Substances Data) |
| Toxizität | LD50 in mice (mg/kg): 115.9 i.p.; 572.3 orally (Palm, Kensler) |