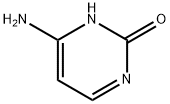
Cytosin
Bezeichnung:Cytosin
CAS-Nr71-30-7
Englisch Name:Cytosine
CBNumberCB3746093
SummenformelC4H5N3O
Molgewicht111.1
MOL-Datei71-30-7.mol
Synonyma
Cytosin
Cytosin physikalisch-chemischer Eigenschaften
| Schmelzpunkt | >300 °C (lit.) |
| Siedepunkt | 208.2°C (rough estimate) |
| Dichte | 0,48 g/cm3 |
| Brechungsindex | 1.5000 (estimate) |
| storage temp. | 2-8°C |
| Löslichkeit | Clear to very slightly hazy colorless to faint yellow solution at 50 mg/ml in 0.5 M HCL. |
| Aggregatzustand | Crystalline Powder |
| pka | 4.60, 12.16(at 25℃) |
| Farbe | White to slightly yellow |
| Biologische Quelle | synthetic (organic) |
| Wasserlöslichkeit | soluble |
| Merck | 14,2795 |
| BRN | 2637 |
| Stabilität | Stable. Incompatible with strong oxidizing agents. |
| Kosmetik-Inhaltsstoffe Funktionen | SKIN CONDITIONING |
| InChI | 1S/C4H5N3O/c5-3-1-2-6-4(8)7-3/h1-2H,(H3,5,6,7,8) |
| InChIKey | OPTASPLRGRRNAP-UHFFFAOYSA-N |
| SMILES | NC1=NC(=O)NC=C1 |
| Kennzeichnung gefährlicher | Xi,Xn |
| R-Sätze: | 36/37/38-20/21/22 |
| S-Sätze: | 26-36-37/39 |
| WGK Germany | 1 |
| RTECS-Nr. | UW7350150 |
| Hazard Note | Irritant |
| TSCA | TSCA listed |
| HS Code | 29335910 |
| Speicherklasse | 11 - Combustible Solids |

