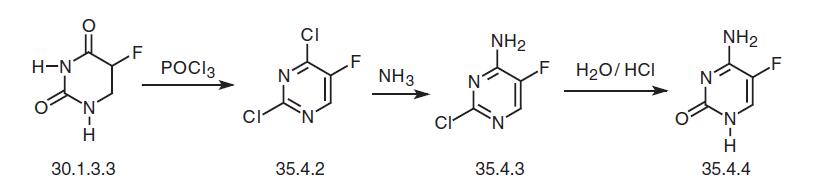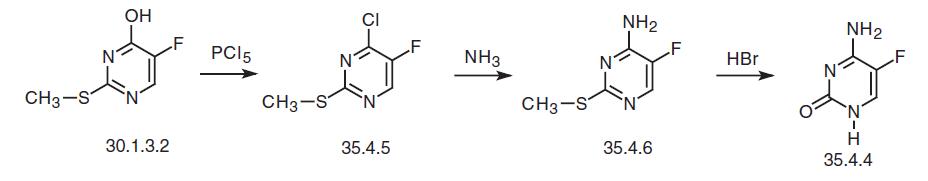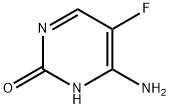
Flucytosin
Bezeichnung:Flucytosin
CAS-Nr2022-85-7
Englisch Name:5-Fluorocytosine
CBNumberCB6744939
SummenformelC4H4FN3O
Molgewicht129.09
MOL-Datei2022-85-7.mol
Synonyma
Flucytosin
Flucytosin physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 298-300 °C (dec.) (lit.) |
| Dichte | 1.3990 (estimate) |
| Dampfdruck | 0Pa at 25℃ |
| storage temp. | 2-8°C |
| Löslichkeit | Sparingly soluble in water, slightly soluble in ethanol (96 per cent) |
| Aggregatzustand | Crystalline Powder |
| pka | 3.26(at 25℃) |
| Farbe | White to almost white |
| Wasserlöslichkeit | 1.5g/100mL (25 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,4125 |
| BRN | 127285 |
| BCS Class | 1 |
| Stabilität | Light Sensitive |
| InChIKey | XRECTZIEBJDKEO-UHFFFAOYSA-N |
| LogP | -1.36 at 22.1℃ and pH6.4-6.9 |
| Dissoziationskonstante | 2.74-10.71 at 21.4℃ |
| CAS Datenbank | 2022-85-7(CAS DataBase Reference) |
| Kennzeichnung gefährlicher | Xn,T,Xi |
| R-Sätze: | 40-36/37/38-63 |
| S-Sätze: | 22-24/25-45-36/37-36/37/39-27-26 |
| WGK Germany | 2 |
| RTECS-Nr. | HA6040000 |
| F | 10-23 |
| Hazard Note | Toxic/Light Sensitive |
| HazardClass | IRRITANT, LIGHT SENSITIVE |
| HS Code | 29335990 |
| Giftige Stoffe Daten | 2022-85-7(Hazardous Substances Data) |
| Toxizität | LD50 in mice (mg/kg): >2000 orally and s.c.; 1190 i.p.; 500 i.v. (Grunberg, 1963) |