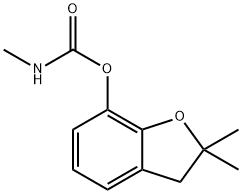
Carbofuran (ISO)
Bezeichnung:Carbofuran (ISO)
CAS-Nr1563-66-2
Englisch Name:Carbofuran
CBNumberCB4322944
SummenformelC12H15NO3
Molgewicht221.25
MOL-Datei1563-66-2.mol
Synonyma
Carbofuran
Carbofuran (ISO)
2,3-Dihydro-2,2-dimethylbenzofuran-7-yl methylcarbamat
Carbofuran (ISO) physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 150-153 °C(lit.) |
| Siedepunkt | 200°C |
| Dichte | 1.18 |
| Dampfdruck | 2 x 10-5 mmHg at 33 °C (quoted, Verschueren, 1983) |
| Brechungsindex | 1.5200 (estimate) |
| storage temp. | 0-6°C |
| Löslichkeit | Methylene chloride (>200 g/L), 2-propanol (20–50 g/L) (Worthing and Hance, 1991) |
| pka | 12.28±0.46(Predicted) |
| Aggregatzustand | Powder |
| Farbe | White, brown |
| Wasserlöslichkeit | Slightly soluble. 0.07 g/100 mL |
| Merck | 13,1813 |
| BRN | 1428746 |
| Henry's Law Constant | 3.88 (x 10-8 atm?m3/mol)at 30 °C (approximate - calculated from water solubility and vapor pressure) |
| Expositionsgrenzwerte | OSHA PEL: TWA 0.1 mg/m3; ACGIH TLV: TWA 0.1 mg/m3. |
| LogP | 2.320 |
| CAS Datenbank | 1563-66-2(CAS DataBase Reference) |
| NIST chemische Informationen | Carbofurane(1563-66-2) |
| Kennzeichnung gefährlicher | T+,N,T |
| R-Sätze: | 26/28-50/53 |
| S-Sätze: | 36/37-45-60-61-1/2 |
| RIDADR | UN 2811 6.1/PG 1 |
| OEB | D |
| OEL | TWA: 0.1 mg/m3 |
| WGK Germany | 3 |
| RTECS-Nr. | FB9450000 |
| HazardClass | 6.1(a) |
| PackingGroup | I |
| HS Code | 29329990 |
| Giftige Stoffe Daten | 1563-66-2(Hazardous Substances Data) |
| Toxizität | LD50 orally in mice: 2 mg/kg (Fahmy, 1970) |


